Complement inhibitors for age-related macular degeneration
- PMID: 37314061
- PMCID: PMC10266126
- DOI: 10.1002/14651858.CD009300.pub3
Complement inhibitors for age-related macular degeneration
Abstract
Background: Age-related macular degeneration (AMD) is a common eye disease and leading cause of sight loss worldwide. Despite its high prevalence and increasing incidence as populations age, AMD remains incurable and there are no treatments for most patients. Mounting genetic and molecular evidence implicates complement system overactivity as a key driver of AMD development and progression. The last decade has seen the development of several novel therapeutics targeting complement in the eye for the treatment of AMD. This review update encompasses the results of the first randomised controlled trials in this field.
Objectives: To assess the effects and safety of complement inhibitors in the prevention or treatment of AMD.
Search methods: We searched CENTRAL on the Cochrane Library, MEDLINE, Embase, LILACS, Web of Science, ISRCTN registry, ClinicalTrials.gov, and the WHO ICTRP to 29 June 2022 with no language restrictions. We also contacted companies running clinical trials for unpublished data.
Selection criteria: We included randomised controlled trials (RCTs) with parallel groups and comparator arms that studied complement inhibition for advanced AMD prevention/treatment.
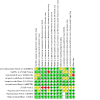
Data collection and analysis: Two authors independently assessed search results and resolved discrepancies through discussion. Outcome measures evaluated at one year included change in best-corrected visual acuity (BCVA), untransformed and square root-transformed geographic atrophy (GA) lesion size progression, development of macular neovascularisation (MNV) or exudative AMD, development of endophthalmitis, loss of ≥ 15 letters of BCVA, change in low luminance visual acuity, and change in quality of life. We assessed risk of bias and evidence certainty using Cochrane risk of bias and GRADE tools.
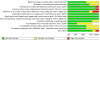
Main results: Ten RCTs with 4052 participants and eyes with GA were included. Nine evaluated intravitreal (IVT) administrations against sham, and one investigated an intravenous agent against placebo. Seven studies excluded patients with prior MNV in the non-study eye, whereas the three pegcetacoplan studies did not. The risk of bias in the included studies was low overall. We also synthesised results of two intravitreal agents (lampalizumab, pegcetacoplan) at monthly and every-other-month (EOM) dosing intervals. Efficacy and safety of IVT lampalizumab versus sham for GA For 1932 participants in three studies, lampalizumab did not meaningfully change BCVA given monthly (+1.03 letters, 95% confidence interval (CI) -0.19 to 2.25) or EOM (+0.22 letters, 95% CI -1.00 to 1.44) (high-certainty evidence). For 1920 participants, lampalizumab did not meaningfully change GA lesion growth given monthly (+0.07 mm², 95% CI -0.09 to 0.23; moderate-certainty due to imprecision) or EOM (+0.07 mm², 95% CI -0.05 to 0.19; high-certainty). For 2000 participants, lampalizumab may have also increased MNV risk given monthly (RR 1.77, 95% CI 0.73 to 4.30) and EOM (RR 1.70, 95% CI 0.67 to 4.28), based on low-certainty evidence. The incidence of endophthalmitis in patients treated with monthly and EOM lampalizumab was 4 per 1000 (0 to 87) and 3 per 1000 (0 to 62), respectively, based on moderate-certainty evidence. Efficacy and safety of IVT pegcetacoplan versus sham for GA For 242 participants in one study, pegcetacoplan probably did not meaningfully change BCVA given monthly (+1.05 letters, 95% CI -2.71 to 4.81) or EOM (-1.42 letters, 95% CI -5.25 to 2.41), as supported by moderate-certainty evidence. In contrast, for 1208 participants across three studies, pegcetacoplan meaningfully reduced GA lesion growth when given monthly (-0.38 mm², 95% CI -0.57 to -0.19) and EOM (-0.29 mm², 95% CI -0.44 to -0.13), with high certainty. These reductions correspond to 19.2% and 14.8% versus sham, respectively. A post hoc analysis showed possibly greater benefits in 446 participants with extrafoveal GA given monthly (-0.67 mm², 95% CI -0.98 to -0.36) and EOM (-0.60 mm², 95% CI -0.91 to -0.30), representing 26.1% and 23.3% reductions, respectively. However, we did not have data on subfoveal GA growth to undertake a formal subgroup analysis. In 1502 participants, there is low-certainty evidence that pegcetacoplan may have increased MNV risk when given monthly (RR 4.47, 95% CI 0.41 to 48.98) or EOM (RR 2.29, 95% CI 0.46 to 11.35). The incidence of endophthalmitis in patients treated with monthly and EOM pegcetacoplan was 6 per 1000 (1 to 53) and 8 per 1000 (1 to 70) respectively, based on moderate-certainty evidence. Efficacy and safety of IVT avacincaptad pegol versus sham for GA In a study of 260 participants with extrafoveal or juxtafoveal GA, monthly avacincaptad pegol probably did not result in a clinically meaningful change in BCVA at 2 mg (+1.39 letters, 95% CI -5.89 to 8.67) or 4 mg (-0.28 letters, 95% CI -8.74 to 8.18), based on moderate-certainty evidence. Despite this, the drug was still found to have probably reduced GA lesion growth, with estimates of 30.5% reduction at 2 mg (-0.70 mm², 95% CI -1.99 to 0.59) and 25.6% reduction at 4 mg (-0.71 mm², 95% CI -1.92 to 0.51), based on moderate-certainty evidence. Avacincaptad pegol may have also increased the risk of developing MNV (RR 3.13, 95% CI 0.93 to 10.55), although this evidence is of low certainty. There were no cases of endophthalmitis reported in this study.
Authors' conclusions: Despite confirmation of the negative findings of intravitreal lampalizumab across all endpoints, local complement inhibition with intravitreal pegcetacoplan meaningfully reduces GA lesion growth relative to sham at one year. Inhibition of complement C5 with intravitreal avacincaptad pegol is also an emerging therapy with probable benefits on anatomical endpoints in the extrafoveal or juxtafoveal GA population. However, there is currently no evidence that complement inhibition with any agent improves functional endpoints in advanced AMD; further results from the phase 3 studies of pegcetacoplan and avacincaptad pegol are eagerly awaited. Progression to MNV or exudative AMD is a possible emergent adverse event of complement inhibition, requiring careful consideration should these agents be used clinically. Intravitreal administration of complement inhibitors is probably associated with a small risk of endophthalmitis, which may be higher than that of other intravitreal therapies. Further research is likely to have an important impact on our confidence in the estimates of adverse effects and may change these. The optimal dosing regimens, treatment duration, and cost-effectiveness of such therapies are yet to be established.
Trial registration: ClinicalTrials.gov NCT02686658 NCT02515942 NCT00935883 NCT01229215 NCT02247479 NCT02247531 NCT01527500 NCT02503332 NCT03525600 NCT03525613 NCT03815825 NCT04435366 NCT04437368 NCT04465955 NCT04566445 NCT04643886 NCT04656561 NCT05019521 NCT05230537.
Copyright © 2023 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
This research did not receive any specific grant from funding agencies in the public, commercial or not‐for‐profit sectors.
Figures
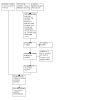
















Update of
-
Complement inhibitors for age-related macular degeneration.Cochrane Database Syst Rev. 2014 Jan 15;2014(1):CD009300. doi: 10.1002/14651858.CD009300.pub2. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2023 Jun 14;6:CD009300. doi: 10.1002/14651858.CD009300.pub3. PMID: 24431152 Free PMC article. Updated.
References
References to studies included in this review
Avacincaptad pegol Phase 2/3 (GATHER1) {published data only (unpublished sought but not used)}
-
- Csaky KG, Westby K, Rezaei K. Avacincaptad pegol, a novel C5 inhibitor, significantly reduces the mean rate of geographic atrophy growth in a pivotal clinical trial. Investigative Ophthalmology and Visual Science 2020;61(7):ARVO E-abstract 1943.
-
- Jaffe GJ, Westby K, Csaky KG, Monés J, Pearlman JA, Patel SS, et al. C5 inhibitor avacincaptad pegol for geographic atrophy due to age-related macular degeneration: a randomized pivotal phase 2/3 trial. Ophthalmology 2021;128(4):576-86. - PubMed
-
- Sheth V. Avacincaptad pegol, a novel C5 inhibitor, demonstrates a continued reduction in the mean rate of geographic atrophy growth: 18-month results from the GATHER1 clinical trial. Investigative Ophthalmology and Visual Science 2021;62(8):ARVO E-abstract 333.
CLG561 ± LFG316 Phase 2 {unpublished data only}
-
- NCT02515942. CLG561 proof-of-concept study as a monotherapy and in combination with LFG316 in subjects with geographic atrophy (GA). clinicaltrials.gov/ct2/show/NCT02515942 (first received 19 December 2018).
Eculizumab Phase 2 (COMPLETE) {published data only}
-
- Garcia Filho CA, Yehoshua Z, Gregori G, Nunes RP, Penha FM, Moshfeghi AA, et al. Change in drusen volume as a novel clinical trial endpoint for the study of complement inhibition in age-related macular degeneration. Ophthalmic Surgery, Lasers and Imaging Retina 2014;45(1):18-31. - PubMed
-
- Yehoshua Z, Amorim Garcia Filho CA, Nunes RP, Gregori G, Penha FM, Moshfeghi AA, et al. Comparison of geographic atrophy growth rates using different imaging modalities in the COMPLETE study. Ophthalmic Surgery, Lasers & Imaging Retina 2015;46(4):413-22. - PubMed
Lampalizumab Phase 2 (MAHALO) {published data only (unpublished sought but not used)}
-
- Hariri A, Nittala MG, Sadda SR. Outer retinal tubulation as a predictor of the enlargement amount of geographic atrophy in age-related macular degeneration. Ophthalmology 2015;122(2):407-13. - PubMed
-
- Kimel M, Leidy NK, Tschosik E, Dolan C, Souied EH, Varma R, et al. Functional Reading Independence (FRI) Index: a new patient-reported outcome measure for patients with geographic atrophy. Investigative Ophthalmology and Visual Science 2016;57(14):6298-304. - PubMed
-
- Sivaprasad S, Tschosik E, Kapre A, Varma R, Bressler NM, Kimel M, et al. Reliability and construct validity of the NEI VFQ-25 in a subset of patients with geographic atrophy from the phase 2 Mahalo Study. American Journal of Ophthalmology 2018;190:1-8. - PubMed
-
- Yaspan BL, Williams DF, Holz FG, Regillo CD, Li Z, Dressen A, et al. Targeting factor D of the alternative complement pathway reduces geographic atrophy progression secondary to age-related macular degeneration. Science Translation Medicine 2017;9:eaaf1443. - PubMed
Lampalizumab Phase 3 (CHROMA) {published data only (unpublished sought but not used)}
-
- Heier JS, Pieramici D, Chakravarthy U, Patel SS, Gupta S, Lotery A, et al. Visual function decline resulting from geographic atrophy: results from the Chroma and Spectri phase 3 trials. Ophthalmology Retina 2020;4(7):673-88. - PubMed
-
- NCT02745119. Long-term safety of lampalizumab intravitreal (ITV) injections in participants with geographic atrophy (GA) secondary to age-related macular degeneration (OMASPECT). clinicaltrials.gov/ct2/show/NCT02745119 (first received 20 April 2016).
-
- Pieramici D, Holz F, Heier JS, Sadda SR, Busbee BG, Chew EY, et al. Lampalizumab for geographic atrophy (GA) in age-related macular degeneration (AMD): pooled results of the Chroma and Spectri phase 3 randomized clinical trials (RCTs). Investigative Ophthalmology and Visual Science 2018;59(9):ARVO E-abstract 4948.
Lampalizumab Phase 3 (SPECTRI) {published data only}
-
- Heier JS, Pieramici D, Chakravarthy U, Patel SS, Gupta S, Lotery A, et al. Visual function decline resulting from geographic atrophy: results from the Chroma and Spectri phase 3 trials. Ophthalmology Retina 2020;4(7):673-88. - PubMed
-
- NCT02745119. Long-term safety of lampalizumab intravitreal (ITV) injections in participants with geographic atrophy (GA) secondary to age-related macular degeneration (OMASPECT) . clinicaltrials.gov/ct2/show/NCT02745119 (first received 20 April 2016).
-
- Pieramici D, Holz F, Heier JS, Sadda SR, Busbee BG, Chew EY, et al. Lampalizumab for geographic atrophy (GA) in age-related macular degeneration (AMD): pooled results of the Chroma and Spectri phase 3 randomized clinical trials (RCTs). Investigative Ophthalmology and Visual Science 2018;59(9):ARVO E-abstract 4948.
LFG316 Phase 2 {unpublished data only}
-
- NCT01527500. Intravitreal LFG316 in patients with age-related macular degeneration (AMD). clinicaltrials.gov/ct2/show/record/NCT01527500 (first received 27 July 2018).
Pegcetacoplan Phase 2 (FILLY) {published and unpublished data}10.1016/j.ophtha.2019.07.011
-
- Bogunovic H, Lachinov D, Mai J, Reiter GS, Riedl S, Vogl WD, et al. Predictive identification of the fastest progressing geographic atrophy lesions based on deep learning in the phase 2 FILLY clinical trial of pegcetacoplan. Investigative Ophthalmology and Visual Science 2021;62(8):ARVO E-abstract 129.
-
- Chakravarthy U, Ip M, Nittala M, Metlapally R, Ribiero R, Sadda S. Impact of pegcetacoplan on progression of nascent atrophy in age-related macular degeneration (AMD). Investigative Ophthalmology and Visual Science 2021;62(8):ARVO E-abstract 1213.
-
- Liao DS, Grossi FV, El Mehdi D, Gerber MR, Brown DM, Heier JS, et al. Complement C3 inhibitor pegcetacoplan for geographic atrophy secondary to age-related macular degeneration: a randomized phase 2 trial. Ophthalmology 2020;127(2):186-95. - PubMed
-
- Liao DS, Metapally R, Joshi P. Pegcetacoplan treatment for geographic atrophy due to age-related macular degeneration: a plain language summary of the FILLY study. Immunotherapy 2022;14(13):995-1006. - PubMed
-
- Nittala MG, Metlapally R, Ip M, Chakravarthy U, Holz FG, Staurenghi G, et al. Association of pegcetacoplan with progression of incomplete retinal pigment epithelium and outer retinal atrophy in age-related macular degeneration: a post hoc analysis of the FILLY randomized clinical trial. JAMA Ophthalmology 2022;140(3):243-9. - PMC - PubMed
Pegcetacoplan Phase 3 (DERBY) {published and unpublished data}
-
- Goldberg R, Heier JS, Wykoff CC, Staurenghi G, Singh RP, Steinle N, et al. Efficacy of intravitreal pegcetacoplan in patients with geographic atrophy (GA): 12-month results from the phase 3 OAKS and DERBY studies. Investigative Ophthalmology and Visual Science 2022;63(7):ARVO E-abstract 1500.
-
- Wykoff CC. Treatment of geographic atrophy secondary to age-related macular degeneration with pegcetacoplan: updates on the randomized phase 3 DERBY and OAKS trials. American Academy of Ophthalmology Annual Meeting, New Orleans, LA, United States 2021.
Pegcetacoplan Phase 3 (OAKS) {published and unpublished data}
-
- Goldberg R, Heier JS, Wykoff CC, Staurenghi G, Singh RP, Steinle N, et al. Efficacy of intravitreal pegcetacoplan in patients with geographic atrophy (GA): 12-month results from the phase 3 OAKS and DERBY studies. Investigative Ophthalmology and Visual Science 2022;63(7):ARVO E-abstract 1500.
-
- Wykoff CC. Treatment of geographic atrophy secondary to age-related macular degeneration with pegcetacoplan: updates on the randomized phase 3 DERBY and OAKS trials. American Academy of Ophthalmology Annual Meeting, New Orleans, LA, United States 2021 2021.
References to studies excluded from this review
NCT01157065 {published data only}
-
- NCT01157065. Evaluation of AL-78898A in exudative age-related macular degeneration (RACE). clinicaltrials.gov/ct2/show/NCT01157065 (first received 5 July 2010).
NCT01624636 {published data only}
-
- NCT01624636. Safety and tolerability of intravenous LFG316 in wet age-related macular degeneration (AMD). clinicaltrials.gov/ct2/show/NCT01624636 (first received 21 June 2012).
NCT03362190 {published data only}
-
- NCT03362190. ZIMURA in combination with LUCENTIS in patients with neovascular age related macular degeneration (NVAMD). clinicaltrials.gov/ct2/show/NCT03362190 (first received 5 December 2017).
NCT03446144 {published data only}
-
- NCT03446144. Safety and efficacy of IONIS-FB-Lrx in up to 120 patients 55 and older with geographic atrophy (GA) secondary to age-related macular degeneration (AMD). clinicaltrials.gov/ct2/show/NCT03446144 (first received 26 February 2018).
References to ongoing studies
NCT03815825 {published data only}
-
- NCT03815825. GOLDEN STUDY: a study to assess safety and efficacy of multiple doses of IONIS-FB-LRx in participants with geographic atrophy secondary to age-related macular degeneration (AMD). clinicaltrials.gov/ct2/show/NCT03815825 (first received 24 January 2019).
NCT04435366 {published data only}
-
- NCT04435366. A phase 3 safety and efficacy study of intravitreal administration of zimura (complement C5 inhibitor). clinicaltrials.gov/ct2/show/NCT04435366 (first received 17 June 2020).
NCT04437368 {published data only}
-
- NCT04437368. EXPLORE: a phase II study to evaluate the safety and efficacy of two doses of GT005 (EXPLORE). clinicaltrials.gov/ct2/show/NCT04437368 (first received 18 June 2020).
NCT04465955 {published data only}
-
- NCT04465955. A study of NGM621 in participants with geographic atrophy (CATALINA). clinicaltrials.gov/ct2/show/NCT04465955 (first received 10 July 2020).
NCT04566445 {published data only}
-
- NCT04566445. HORIZON: a phase II study to evaluate the safety and efficacy of two doses of GT005. clinicaltrials.gov/ct2/show/NCT04566445 (first received 28 September 2020).
NCT04643886 {published data only}
-
- NCT04643886. A multiple dose study of repeat intravitreal injections of GEM103 in dry age-related macular degeneration. clinicaltrials.gov/ct2/show/NCT04643886 (first received 25 November 2020).
NCT04656561 {published data only}
-
- NCT04656561. A study investigating the efficacy and safety of intravitreal injections of ANX007 in patients with geographic atrophy (ARCHER). clinicaltrials.gov/ct2/show/NCT04656561 (first received 7 December 2020).
NCT04820452 {published data only}
-
- NCT04820452. A study of IBI302 in patients with nAMD. clinicaltrials.gov/ct2/show/NCT04820452 (first received 29 March 2021).
NCT05019521 {published data only}
-
- NCT05019521. A study of danicopan in participants with geographic atrophy secondary to age-related macular degeneration. clinicaltrials.gov/ct2/show/NCT05019521 (first received 25 August 2021).
NCT05230537 {published data only}
-
- NCT05230537. A masked, placebo-controlled study to assess iptacopan in age-related macular degeneration. clinicaltrials.gov/ct2/show/NCT05230537 (first received 9 February 2022).
Additional references
Anderson 2010
Augustin 2007
-
- Augustin A, Sahel JA, Bandello F, Dardennes R, Maurel F, Negrini C, et al. Anxiety and depression prevalence rates in age-related macular degeneration. Investigative Ophthalmology and Visual Science 2007;48(4):1498-503. - PubMed
Balaskas 2022
Beck 2007
-
- Beck RW, Maguire MG, Bressler NM, Glassman AR, Lindblad AS, Ferris FL. Visual acuity as an outcome measure in clinical trials of retinal diseases. Ophthalmology 2007;114(10):1804-9. - PubMed
Booij 2010
-
- Booij JC, Baas DC, Beisekeeva J, Gorgels TG, Bergen AA. The dynamic nature of Bruch's membrane. Progress in Retinal and Eye Research 2010;29(1):1-18. - PubMed
Bora 2005
-
- Bora PS, Sohn JH, Cruz JM, Jha P, Nishihori H, Wang Y, et al. Role of complement and complement membrane attack complex in laser-induced choroidal neovascularization. Journal of Immunology 2005;174(1):491-7. - PubMed
Boutron 2022
-
- Boutron I, Page MJ, Higgins JPT, Altman DG, Lundh A, Hróbjartsson A. Chapter 7: Considering bias and conflicts of interest among the included studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Brechner 2011
-
- Brechner RJ, Rosenfeld PJ, Babish JD, Caplan S. Pharmacotherapy for neovascular age-related macular degeneration: an analysis of the 100% 2008 medicare fee-for-service part B claims file. American Journal of Ophthalmology 2011;151:887-95. - PubMed
Brown 1999
Brown 2020
-
- Brown GC, Brown MM, Rapuano S, Boyer D. Cost-utility analysis of VEGF inhibitors for treating neovascular age-related macular degeneration. American Journal of Ophthalmology 2020;218:225-41. - PubMed
Calippe 2017
-
- Calippe B, Augustin S, Beguier F, Charles-Messance H, Poupel L, Conart JB, et al. Complement factor H inhibits CD47-mediated resolution of inflammation. Immunity 2017;46(2):261-72. - PubMed
Cao 2011
Cao 2021
-
- Cao D, Leong B, Messinger JD, Kar D, Ach T, Yannuzzi LA, et al. Hyperreflective foci, optical coherence tomography progression indicators in age-related macular degeneration, include transdifferentiated retinal pigment epithelium. Investigative Ophthalmology and Visual Science 2021;62(10):ARVO E-abstract 34. - PMC - PubMed
Cerniauskas 2020
Chakravarthy 2010
Chakravarthy 2018
-
- Chakravarthy U, Bailey CC, Johnston RL, McKibbin M, Khan RS, Mahmood S, et al. Characterizing disease burden and progression of geographic atrophy secondary to age-related macular degeneration. Ophthalmology 2018;125(6):842-9. - PubMed
Chia 2004
-
- Chia EM, Wang JJ, Rochtchina E, Smith W, Cumming RR, Mitchell P. Impact of bilateral visual impairment on health-related quality of life: the Blue Mountains Eye Study. Investigative Ophthalmology and Visual Science 2004;45(1):71-6. - PubMed
Cocce 2018
Coleman 2008
-
- Coleman AL, Yu F. Eye-related medicare costs for patients with age-related macular degeneration from 1995 to 1999. Ophthalmology 2008;115(1):18-25. - PubMed
Colijn 2017
Colijn 2021
Copland 2018
-
- Copland DA, Theodoropoulou S, Liu J, Dick AD. A perspective of AMD through the eyes of immunology. Investigative Ophthalmology and Visual Science 2018;59(4):AMD83-92. - PubMed
Covidence [Computer program]
-
- Covidence. Melbourne, Australia: Veritas Health Innovation, accessed 1 October 2022. Available at www.covidence.org.
Creuzot‐Garcher 2022
Csaky 2017
Deeks 2022
-
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022 . Available from www.training.cochrane.org/handbook.
Despriet 2006
-
- Despriet DD, Klaver CC, Witteman JC, Bergen AA, Kardys I, Maat MP, et al. Complement factor H polymorphism, complement activators, and risk of age-related macular degeneration. JAMA 2006;296(3):301-9. - PubMed
Duvall‐Young 1989
Edwards 2005
-
- Edwards AO, Ritter R 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science 2005;308(5720):421-4. - PubMed
Ferris 2013
Feuer 2013
-
- Feuer WJ, Yehoshua Z, Gregori G, Penha FM, Chew EY, Ferris FL, et al. Square root transformation of geographic atrophy area measurements to eliminate dependence of growth rates on baseline lesion measurements: a reanalysis of age-related eye disease study report no. 26. JAMA Ophthalmology 2013;131(1):110-1. - PMC - PubMed
Flaxman 2017
-
- Flaxman SR, Bourne RR, Resnikoff S, Ackland P, Braithwaite T, Cicinelli MV, et al. Global causes of blindness and distance vision impairment 1990–2020: A systematic review and meta-analysis. Lancet Global Health 2017;5(12):1221-34. - PubMed
Fleckenstein 2018
-
- Fleckenstein M, Mitchell P, Freund KB, Sadda S, Holz FG, Brittain C, et al. The progression of geographic atrophy secondary to age-related macular degeneration. Ophthalmology 2018;125(3):369-90. - PubMed
Fritsche 2016
Galimard 2018
Gold 2006
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), accessed 24 September 2022. Available at gradepro.org.
Guillonneau 2017
-
- Guillonneau X, Eandi CM, Paques M, Sahel JA, Sapieha P, Sennlaub F. On phagocytes and macular degeneration. Progress in Retinal and Eye Research 2017;61:98-128. - PubMed
Gupta 2007
-
- Gupta OP, Brown GC, Brown MM. Age-related macular degeneration: the costs to society and the patient. Current Opinion in Ophthalmology 2007;18(3):201-5. - PubMed
Gupta 2019
Hageman 2005
-
- Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proceedings of the National Academy of Sciences of the United States of America 2005;102(20):7227-32. - PMC - PubMed
Haines 2005
-
- Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science 2005;308(5720):419-21. - PubMed
Hallam 2020
-
- Hallam TM, Marchbank KJ, Harris CL, Osmond C, Shuttleworth VG, Griffiths H, et al. Rare genetic variants in complement factor I lead to low FI plasma levels resulting in increased risk of age-related macular degeneration. Investigative Ophthalmology and Visual Science 2020;61(6):ARVO E-abstract 18. - PMC - PubMed
Harboe 2004
Harrell 2015
-
- Harrell FE. Multivariable Modeling Strategies. In: Regression Modeling Strategies. Springer Series in Statistics. Springer Cham, 2015. [DOI: 10.1007/978-3-319-19425-7_4] - DOI
Hassell 2006
Heesterbeek 2020
Heier 2020
-
- Heier JS, Pieramici D, Chakravarthy U, Patel SS, Gupta S, Lotery A, et al. Visual function decline resulting from geographic atrophy: results from the Chroma and Spectri phase 3 trials. Ophthalmology Retina 2020;4(7):673-88. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Higgins 2019
-
- Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Hillmen 2021
-
- Hillmen P, Szer J, Weitz I, Röth A, Höchsmann B, Panse J, et al. Pegcetacoplan versus eculizumab in paroxysmal nocturnal hemoglobinuria. New England Journal of Medicine 2021;384:1028-37. - PubMed
Hodge 2013
-
- Hodge S, Eccles F. Loneliness, Social Isolation and Sight Loss: A literature review conducted for Thomas Pocklington Trust. Lancaster University. eprints.lancs.ac.uk/id/eprint/68597/1/loneliness_social_isolation_and_si... accessed 7 October 2022.
Hoh Kam 2013
-
- Hoh Kam J, Lenassi E, Malik TH, Pickering MC, Jeffery G. Complement component C3 plays a critical role in protecting the aging retina in a murine model of age-related macular degeneration. American Journal of Pathology 2013;183(2):480-92. - PubMed
Hughes 2019
Irmscher 2018
Jacob 2017
Kahr 2010
-
- Kahr WH. Complement halts angiogenesis gone wild. Blood 2010;116(22):4393-4. - PubMed
Karczewski 2020
Katschke 2012
Keenan 2012
Keenan 2018
Kenward 2007
-
- Kenward MG, Carpenter J. Multiple imputation: current perspectives. Statistical Methods in Medical Research 2007;16(3):199-218. - PubMed
Kim 2021
Kirkham 2018
Klein 2005
Lee 2010
-
- Lee HM, Wysoczynski M, Liu R, Shin DM, Kucia M, Botto M, et al. Mobilization studies in complement-deficient mice reveal that optimal AMD3100 mobilization of hematopoietic stem cells depends on complement cascade activation by AMD3100-stimulated granulocytes. Leukemia 2010;24(3):573-82. - PMC - PubMed
Li 2019
-
- Li JQ, Welchowski T, Schmid M, Mauschitz MM, Holz FG, Finger RP. Prevalence and incidence of age-related macular degeneration in Europe: a systematic review and meta-analysis. British Journal of Ophthalmology 2020;104(8):1077-84. - PubMed
Lindblad 2009
Litts 2015
-
- Litts KM, Messinger JD, Dellatorre K, Yannuzzi LA, Freund KB, Curcio CA. Clinicopathological correlation of outer retinal tubulation in age-related macular degeneration. JAMA Ophthalmology 2015;133(5):609-12. - PubMed
Lorés‐Motta 2018
-
- Lorés-Motta L, Paun CC, Corominas J, Pauper M, Geerlings MJ, Altay L, et al. Genome-wide association study reveals variants in CFH and CFHR4 associated with systemic complement activation: Implications in age-related macular degeneration. Ophthalmology 2018;125(7):1064-74. - PubMed
Lyzogubov 2011
Mastellos 2013
Mastellos 2019
Meleth 2011
Minassian 2009
-
- Minassian D, Reidy A. Future Sight Loss UK 2: An epidemiological and economic model for sight loss in the decade 2010-2020. www.rnib.org.uk/professionals/health-social-care-education-professionals... (accessed 31 August 2022).
Mohlin 2017
-
- Mohlin C, Sandholm K, Ekdahl KN, Nilsson B. The link between morphology and complement in ocular disease. Molecular Immunology 2017;89:84-99. - PubMed
Morgan 2016
-
- Morgan BP. The membrane attack complex as an inflammatory trigger. Immunobiology 2016;221(6):747-51. - PubMed
Mulligan 2019
Nittala 2022
-
- Nittala MG, Metlapally R, Ip M, Chakravarthy U, Holz FG, Staurenghi G, et al. Association of pegcetacoplan with progression of incomplete retinal pigment epithelium and outer retinal atrophy in age-related macular degeneration: a post hoc analysis of the FILLY randomized clinical trial. JAMA Ophthalmology 2022;140(3):243-9. - PMC - PubMed
Nozaki 2006
Raychaudhuri 2011
RevMan Web 2022 [Computer program]
-
- Review Manager Web (RevMan Web). The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Riley 2011
-
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ 2011;342:594. - PubMed
Rofagha 2013
-
- Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K, SEVEN-UP Study Group. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: A multicenter cohort study (SEVEN-UP). Ophthalmology 2013;120(11):2292-9. - PubMed
Ruan 2015
-
- Ruan CC, Ge Q, Li Y, Li XD, Chen DR, Ji KD, et al. Complement-mediated macrophage polarization in perivascular adipose tissue contributes to vascular injury in deoxycorticosterone acetate-salt mice. Arteriosclerosis, Thrombosis, and Vascular Biology 2015;35(3):598-606. - PubMed
Sarks 1988
-
- Sarks JP, Sarks SH, Killingsworth MC. Evolution of geographic atrophy of the retinal pigment epithelium. Eye 1988;2(Pt 5):552-77. - PubMed
Schaal 2015
Schmier 2012
-
- Schmier JK, Covert DW, Lau EC. Patterns and costs associated with progression of age-related macular degeneration. American Journal of Ophthalmology 2012;154:675-81. - PubMed
Schmitz‐Valckenberg 2016
-
- Schmitz-Valckenberg S, Nadal J, Fimmers R, Lindner M, Holz FG, Schmid M, et al. Modeling visual acuity in geographic atrophy secondary to age-related macular degeneration. Ophthalmologica 2016;235(4):215-24. - PubMed
Seddon 2013
Shahid 2012
-
- Shahid H, Khan JC, Cipriani V, Sepp T, Matharu BK, Bunce C, et al. Age-related macular degeneration: the importance of family history as a risk factor. British Journal of Ophthalmology 2012;96(3):427-31. - PubMed
Sharma 2000
-
- Sharma S, Brown GC, Brown MM, Shah GK, Snow K, Brown H, et al. Converting visual acuity to utilities. Canadian Journal of Ophthalmology 2000;35(5):267-72. - PubMed
Shaw 2012
Simkiss 2016
-
- Simkiss P, Dennison D, Edwards E, Edwards R, Flynn K, Lee H, et al. The State of the Nation Eye Health 2016. RNIB. www.rnib.org.uk/stateofthenation (accessed 31 August 2022).
Slakter 2005
-
- Slakter JS, Stur M. Quality of life in patients with age-related macular degeneration: impact of the condition and benefits of treatment. Survey of Ophthalmology 2005;50(3):263-73. - PubMed
Soubrane 2007
-
- Soubrane G, Cruess A, Lotery A, Pauleikhoff D, Monès J, Xu X, et al. Burden and health care resource utilization in neovascular age-related macular degeneration: findings of a multicountry study. Archives of Ophthalmology 2007;125(9):1249-54. - PubMed
Spencer 2007
-
- Spencer KL, Hauser MA, Olson LM, Schmidt S, Scott WK, Gallins P, et al. Protective effect of complement factor B and complement component 2 variants in age-related macular degeneration. Human Molecular Genetics 2007;16(16):1986-92. - PubMed
Spratt 2010
-
- Spratt M, Carpenter J, Sterne JA, Carlin JB, Heron J, Henderson J, et al. Strategies for multiple imputation in longitudinal studies. American Journal of Epidemiology 2010;172(4):478-87. - PubMed
Steinle 2021
-
- Steinle NC, Pearce I, Monés J, Metlapally R, Saroj N, Hamdani M, et al. Impact of baseline characteristics on geographic atrophy progression in the FILLY trial evaluating the complement C3 inhibitor pegcetacoplan. American Journal of Ophthalmology 2021;227:116-24. - PubMed
Submacular Surgery Trials Research Group 2007
-
- Submacular Surgery Trials Research Group. Evaluation of minimum clinically meaningful changes in scores on the National Eye Institute Visual Function Questionnaire (NEI-VFQ) SST Report Number 19. Ophthalmic Epidemiology 2007;14(4):205-15. - PubMed
Sunness 1999
-
- Sunness JS, Gonzalez-Baron J, Applegate CA, Bressler NM, Tian Y, Hawkins B, et al. Enlargement of atrophy and visual acuity loss in the geographic atrophy form of age-related macular degeneration. Ophthalmology 1999;106(9):1768-79. - PubMed
Sunness 2005
Tzoumas 2021
-
- Tzoumas N, Hallam D, Harris CL, Lako M, Kavanagh D, Steel DH. Revisiting the role of factor H in age-related macular degeneration: Insights from complement-mediated renal disease and rare genetic variants. Survey of Ophthalmology 2021;66(2):378-401. - PubMed
Tzoumas 2022
Undar 2002
-
- Undar A, Eichstaedt HC, Clubb FJ Jr, Fung M, Lu M, Bigley JE, et al. Novel anti-factor D monoclonal antibody inhibits complement and leukocyte activation in a baboon model of cardiopulmonary bypass. Annals of Cardiothoracic Surgery 2002;74(2):355-62. - PubMed
van Asten 2018
van de Ven 2013
-
- de Ven JP, Nilsson SC, Tan PL, Buitendijk GH, Ristau T, Mohlin FC, et al. A functional variant in the CFI gene confers a high risk of age-related macular degeneration. Nature Genetics 2013;45(7):813-7. - PubMed
VanderBeek 2015
Vu 2005
Weismann 2011
Whitmore 2015
Winkler 2020
Wittenborn 2013
Wong 2014
-
- Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Global Health 2014;2(2):e106-16. - PubMed
Wu 2018
Wykoff 2021
-
- Wykoff CC, Rosenfeld PJ, Waheed NK, Singh RP, Ronca N, Slakter JS, et al. Characterizing new-onset exudation in the randomized phase 2 FILLY trial of complement inhibitor pegcetacoplan for geographic atrophy. Ophthalmology 2021;128(9):1325-36. - PubMed
Yanamadala 2010
Yates 2007
-
- Yates JR, Sepp T, Matharu BK, Khan JC, Thurlby DA, Shahid H, et al. Complement C3 variant and the risk of age-related macular degeneration. New England Journal of Medicine 2007;357(6):553-61. - PubMed
Zelek 2019
-
- Zelek WM, Xie L, Morgan BP, Harris CL. Compendium of current complement therapeutics. Molecular Immunology 2019;114:341-52. - PubMed
References to other published versions of this review
Williams 2011
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

