Subcutaneous abatacept for the treatment of rheumatoid arthritis in routine clinical practice in Germany, Austria, and Switzerland: 2-year retention and efficacy by treatment line and serostatus
- PMID: 37314665
- PMCID: PMC10412468
- DOI: 10.1007/s10067-023-06649-x
Subcutaneous abatacept for the treatment of rheumatoid arthritis in routine clinical practice in Germany, Austria, and Switzerland: 2-year retention and efficacy by treatment line and serostatus
Abstract
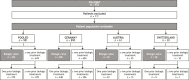
Introduction/objectives: The ASCORE study on treatment for rheumatoid arthritis (RA) showed better retention and clinical response rates for abatacept as first-line versus later-line therapy. This post hoc analysis of ASCORE assessed 2-year retention, efficacy, and safety of subcutaneous (SC) abatacept in Germany, Austria, and Switzerland.
Methods: Adults with RA who initiated SC abatacept 125 mg once weekly were assessed. Primary endpoint was abatacept retention rate at 2 years. Secondary endpoints: proportions of patients with low disease activity (LDA)/remission per Disease Activity Score in 28 joints based on erythrocyte sedimentation rate (≤ 3.2), Simplified Disease Activity Index (≤ 11), and Clinical Disease Activity Index (≤ 10). Outcomes were analyzed by treatment line and serostatus.
Results: For the pooled cohort, the 2-year abatacept retention rate was 47.6%; retention was highest in biologic-naïve patients (50.5% [95% confidence interval 44.9, 55.9]). Patients seropositive for both anti-citrullinated protein antibody (ACPA) and rheumatoid factor (RF; + / +) at baseline had a higher 2-year abatacept retention rate than patients with single seropositivity for either APCA or RF or double-seronegativity (- / -), irrespective of treatment line. At 2 years, a higher proportion of patients who were biologic-naïve were in LDA/remission than patients with one or ≥ two prior biologics.
Conclusion: A higher proportion of patients with + / + RA (compared with - / - RA) had abatacept retention after 2 years. Early identification of patients with seropositive RA may facilitate a precision-medicine approach to RA treatment, leading to a higher proportion of patients in LDA/remission.
Trial registration number: NCT02090556; date registered: March 18, 2014 (retrospectively registered). Key Points • This post hoc analysis of a German-speaking subset of European patients with RA from the global ASCORE study (NCT02090556) showed that retention of SC abatacept within this subset was 47.6%, with good clinical outcomes after 2 years. • Patients with double-seropositive RA (ACPA and RF positive) had higher retention of abatacept than patients with double-seronegative RA (ACPA and RF negative). Retention and clinical responses were highest for patients who were biologic-naïve compared with patients who had one or ≥ two prior biologic treatments. • These real-world data may be useful for clinicians in informing individualized treatment pathways for patients with RA, and fostering superior disease control and clinical outcomes.
Keywords: Abatacept; Clinical response; Retention; Rheumatoid arthritis; Serostatus; bDMARD.
© 2023. The Author(s).
Conflict of interest statement
RA has received consulting fees from AbbVie, Bristol Myers Squibb, Celltrion, Galapagos, Gilead, Janssen, Lilly, Novartis, Pfizer, and Roche; and has received grant/research support from Bristol Myers Squibb, Galapagos, Gilead, Janssen, Lilly, Novartis, Pfizer, and Roche. BB, PP, AF, and HN declare no competing interests. CR (at time of analysis), MC, SEC, RH, and KL are employees of and shareholders in Bristol Myers Squibb. H-PT has received consulting fees from AbbVie, Bristol Myers Squibb, Chugai, Galapagos, Gilead, Janssen, Lilly, Novartis, and Roche.
Figures



References
-
- Katchamart W, Koolvisoot A, Aromdee E, Chiowchanwesawakit P, Muengchan C. Associations of rheumatoid factor and anti-citrullinated peptide antibody with disease progression and treatment outcomes in patients with rheumatoid arthritis. Rheumatol Int. 2015;35:1693–1699. doi: 10.1007/s00296-015-3271-8. - DOI - PubMed
-
- Alemao E, Bao Y, Weinblatt ME, Shadick N. Association of Seropositivity and Mortality in Rheumatoid Arthritis and the Impact of Treatment With Disease-Modifying Antirheumatic Drugs: Results From a Real-World Study. Arthritis Care Res (Hoboken) 2020;72:176–183. doi: 10.1002/acr.24071. - DOI - PMC - PubMed
-
- Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, 3rd, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B, Costenbader KH, Dougados M, Emery P, Ferraccioli G, Hazes JM, Hobbs K, Huizinga TW, Kavanaugh A, Kay J, Kvien TK, Laing T, Mease P, Menard HA, Moreland LW, Naden RL, Pincus T, Smolen JS, Stanislawska-Biernat E, Symmons D, Tak PP, Upchurch KS, Vencovsky J, Wolfe F, Hawker G. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–2581. doi: 10.1002/art.27584. - DOI - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

