Cost-effectiveness of FOLFOX6+Bevacizumab Versus FOLFOX6+Cetuximab in Stage IV Colorectal Cancer Patients in Shiraz, Iran
- PMID: 37314727
- PMCID: PMC10286541
- DOI: 10.1177/10732748231180679
Cost-effectiveness of FOLFOX6+Bevacizumab Versus FOLFOX6+Cetuximab in Stage IV Colorectal Cancer Patients in Shiraz, Iran
Abstract
Background: Colorectal cancer is one of the most common cancers in the world, with about one million cases diagnosed annually. Various treatment methods can be used to treat colorectal cancer, including chemotherapy with different drug regimens. Considering the need to opt for more effective and less expensive drugs in the treatment of this disease, the present study aimed to compare the cost-effectiveness of FOLFOX6+Bevacizumab with FOLFOX6+Cetuximab in patients with stage IV colorectal cancer referred to medical centers in Shiraz, Iran, in 2021.
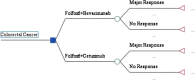
Materials and methods: Using a decision tree, the cost-effectiveness and cost-utility of the 2 drug regimens were compared in all studied patients through the census method. Having a societal perspective, this study considered direct medical costs, direct non-medical costs, and indirect costs. The effectiveness indicators included the rate of major response to the drug combination used and the Quality-adjusted Life Year (QALY). The data were analyzed using Treeage 2011 and Excel 2016 software. In order to ensure the robustness of the results, one-way and probabilistic sensitivity analyses were performed as well.
Results: The results showed that the expected costs, the effectiveness (major response rate), and the QALYs of the FOLFOX6+Bevacizumab drug regimen were $16746.13(USD), .49, and .19, respectively, and those of the FOLFOX6+Cetuximab regimen were, respectively, $15191.05 (USD), .68, and .22. Therefore, FOLFOX6+Cetuximab compared to FOLFOX6+Bevacizumab was less costly and more effective and had a greater QALY, thus being considered as the dominant option. Also, the results of the sensitivity analyses showed that there was a bit of uncertainty.
Conclusion: Considering that the FOLFOX6+Cetuximab regimen was more cost-effective, it is suggested to be prioritized in preparing clinical guidelines for Iranian colorectal cancer patients. In addition, increasing the basic and supplementary insurance coverage for this drug combination as well as the use of remote technology to guide patients by oncologists can be solutions to reduce direct and indirect costs of the patients.
Keywords: Bevacizumab; Cetuximab; FOLFOX6; colorectal cancer; cost-effectiveness.
Conflict of interest statement
RR, AJ, and MD contributed to the idea and design. SSTF contributed to the data collection. RR, AJ, and SSTF contributed to the data analysis. All authors contributed to the manuscript writing and revision. All authors approved the final version of the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures



Similar articles
-
A Cost-effectiveness Analysis of Adding Cetuximab to the First-line Treatment of Metastatic Colorectal Carcinoma in Iran; Considering Genetic Screening for Precision Medicine.J Gastrointest Cancer. 2023 Dec;54(4):1212-1219. doi: 10.1007/s12029-022-00904-1. Epub 2023 Jan 9. J Gastrointest Cancer. 2023. PMID: 36622516
-
Cost-effectiveness of FOLFIRI + cetuximab vs FOLFIRI + bevacizumab in the first-line treatment of RAS wild-type metastatic colorectal cancer in Germany: data from the FIRE-3 (AIO KRK-0306) study.J Med Econ. 2020 May;23(5):448-455. doi: 10.1080/13696998.2019.1709848. Epub 2020 Jan 17. J Med Econ. 2020. PMID: 31903807
-
Cost-Effectiveness Analysis of First-Line FOLFIRI Combined With Cetuximab or Bevacizumab in Patients With RAS Wild-Type Left-Sided Metastatic Colorectal Cancer.Cancer Control. 2020 Jan-Dec;27(1):1073274820902271. doi: 10.1177/1073274820902271. Cancer Control. 2020. PMID: 32107929 Free PMC article. Clinical Trial.
-
Economic Evaluation of Monoclonal Antibodies in Metastatic Colorectal Cancer: A Systematic Review.Mol Diagn Ther. 2021 Nov;25(6):715-734. doi: 10.1007/s40291-021-00560-4. Epub 2021 Nov 24. Mol Diagn Ther. 2021. PMID: 34816395
-
Integration of novel agents in the treatment of colorectal cancer.Cancer Chemother Pharmacol. 2004 Sep;54 Suppl 1:S32-9. doi: 10.1007/s00280-004-0884-0. Cancer Chemother Pharmacol. 2004. PMID: 15309512 Review.
Cited by
-
Decision-Analytical Modelling of Medicines in the Middle East: A Systematic Review of Economic Evaluation Studies.Appl Health Econ Health Policy. 2025 Jul;23(4):569-612. doi: 10.1007/s40258-024-00940-x. Epub 2025 Apr 12. Appl Health Econ Health Policy. 2025. PMID: 40221639
References
-
- Iannazzo S, Distante C, Lopatriello S, Bordonaro R. A cost comparison of biologic treatment regimens for metastatic colorectal cancer in Italy. Global and Regional Health Technology Assessment. 2017;4(1):221-226.
-
- Parkin D, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA A Cancer J Clin. 2005;55(2):74-108. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

