Acceptability, Tolerability, and Estimates of Putative Treatment Effects of Probiotics as Adjunctive Treatment in Patients With Depression: A Randomized Clinical Trial
- PMID: 37314797
- PMCID: PMC10267847
- DOI: 10.1001/jamapsychiatry.2023.1817
Acceptability, Tolerability, and Estimates of Putative Treatment Effects of Probiotics as Adjunctive Treatment in Patients With Depression: A Randomized Clinical Trial
Abstract
Importance: The microbiota-gut-brain axis is a promising target for novel treatments for mood disorders, such as probiotics. However, few clinical trials have been conducted, and further safety and efficacy data are needed to support this treatment approach.
Objective: To provide acceptability and tolerability data and estimates of intervention effect size for probiotics as adjunctive treatment for patients with major depressive disorder (MDD).
Design, setting, and participants: In this single-center, double-blind, placebo-controlled pilot randomized clinical trial, adults aged 18 to 55 years with MDD taking antidepressant medication but having an incomplete response were studied. A random sample was recruited from primary and secondary care services and general advertising in London, United Kingdom. Data were collected between September 2019 and May 2022 and analyzed between July and September 2022.
Intervention: Multistrain probiotic (8 billion colony-forming units per day) or placebo daily for 8 weeks added to ongoing antidepressant medication.
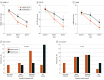
Main outcomes and measures: The pilot outcomes of the trial were retention, acceptability, tolerability, and estimates of putative treatment effect on clinical symptoms (depression: Hamilton Depression Rating Scale [HAMD-17] and Inventory of Depressive Symptomatology [IDS] scores; anxiety: Hamilton Anxiety Rating Scale [HAMA] and General Anxiety Disorder [GAD-7] scores) to be used as indicators for a definitive trial.
Results: Of 50 included participants, 49 received the intervention and were included in intent-to-treat analyses; of these, 39 (80%) were female, and the mean (SD) age was 31.7 (9.8) years. A total of 24 were randomized to probiotic and 25 to placebo. Attrition was 8% (1 in the probiotic group and 3 in the placebo group), adherence was 97.2%, and there were no serious adverse reactions. For the probiotic group, mean (SD) HAMD-17 scores at weeks 4 and 8 were 11.00 (5.13) and 8.83 (4.28), respectively; IDS, 30.17 (11.98) and 25.04 (11.68); HAMA, 11.71 (5.86) and 8.17 (4.68); and GAD-7, 7.78 (4.12) and 7.63 (4.77). For the placebo group, mean (SD) HAMD-17 scores at weeks 4 and 8 were 14.04 (3.70) and 11.09 (3.22), respectively; IDS, 33.82 (9.26) and 29.64 (9.31); HAMA, 14.70 (5.47) and 10.95 (4.48); and GAD-7, 10.91 (5.32) and 9.48 (5.18). Standardized effect sizes (SES) from linear mixed models demonstrated that the probiotic group attained greater improvements in depressive symptoms according to HAMD-17 scores (week 4: SES, 0.70; 95% CI, 0.01-0.98) and IDS Self Report scores (week 8: SES, 0.64; 95% CI, 0.03-0.87) as well as greater improvements in anxiety symptoms according to HAMA scores (week 4: SES, 0.67; 95% CI, 0-0.95; week 8: SES, 0.79; 95% CI, 0.06-1.05), but not GAD-7 scores (week 4: SES, 0.57; 95% CI, -0.01 to 0.82; week 8: SES, 0.32; 95% CI, -0.19 to 0.65), compared with the placebo group.
Conclusions and relevance: The acceptability, tolerability, and estimated effect sizes on key clinical outcomes are promising and encourage further investigation of probiotics as add-on treatment for people with MDD in a definitive efficacy trial.
Trial registration: ClinicalTrials.gov Identifier: NCT03893162.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

