Recent Advances in Single-Molecule Tracking and Imaging Techniques
- PMID: 37314878
- PMCID: PMC11729782
- DOI: 10.1146/annurev-anchem-091922-073057
Recent Advances in Single-Molecule Tracking and Imaging Techniques
Abstract
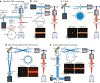
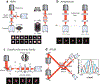
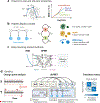
Since the early 1990s, single-molecule detection in solution at room temperature has enabled direct observation of single biomolecules at work in real time and under physiological conditions, providing insights into complex biological systems that the traditional ensemble methods cannot offer. In particular, recent advances in single-molecule tracking techniques allow researchers to follow individual biomolecules in their native environments for a timescale of seconds to minutes, revealing not only the distinct pathways these biomolecules take for downstream signaling but also their roles in supporting life. In this review, we discuss various single-molecule tracking and imaging techniques developed to date, with an emphasis on advanced three-dimensional (3D) tracking systems that not only achieve ultrahigh spatiotemporal resolution but also provide sufficient working depths suitable for tracking single molecules in 3D tissue models. We then summarize the observables that can be extracted from the trajectory data. Methods to perform single-molecule clustering analysis and future directions are also discussed.
Keywords: clustering analysis; feedback-control tracking; multiple plane microscopy; point-spread-function engineering; single-molecule tracking; trajectory analysis.
Figures


References
-
- Dovichi NJ, Martin JC, Jett JH, Trkula M, Keller RA. 1984. Laser-induced fluorescence of flowing samples as an approach to single-molecule detection in liquids. Anal. Chem 56:348–54 - PubMed
-
- Keller RA, Ambrose WP, Goodwin PM, Jett JH, Martin JC, Wu M. 1996. Single-molecule fluorescence analysis in solution. Appl. Spectrosc 50:12A–32A
-
- Shera EB, Seitzinger NK, Davis LM, Keller RA, Soper SA. 1990. Detection of single fluorescent molecules. Chem. Phys. Lett 174:553–57
-
- Soper SA, Shera EB, Martin JC, Jett JH, Hahn JH, et al. 1991. Single-molecule detection of Rhodamine 6G in ethanolic solutions using continuous wave laser excitation. Anal. Chem 63:432–37
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

