Breast cancer cell mesenchymal transition and metastasis directed by DAP5/eIF3d-mediated selective mRNA translation
- PMID: 37314929
- PMCID: PMC10895648
- DOI: 10.1016/j.celrep.2023.112646
Breast cancer cell mesenchymal transition and metastasis directed by DAP5/eIF3d-mediated selective mRNA translation
Abstract
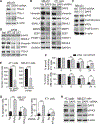
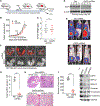
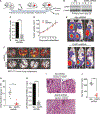
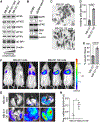
Cancer cell plasticity enables cell survival in harsh physiological environments and fate transitions such as the epithelial-to-mesenchymal transition (EMT) that underlies invasion and metastasis. Using genome-wide transcriptomic and translatomic studies, an alternate mechanism of cap-dependent mRNA translation by the DAP5/eIF3d complex is shown to be essential for metastasis, EMT, and tumor directed angiogenesis. DAP5/eIF3d carries out selective translation of mRNAs encoding EMT transcription factors and regulators, cell migration integrins, metalloproteinases, and cell survival and angiogenesis factors. DAP5 is overexpressed in metastatic human breast cancers associated with poor metastasis-free survival. In human and murine breast cancer animal models, DAP5 is not required for primary tumor growth but is essential for EMT, cell migration, invasion, metastasis, angiogenesis, and resistance to anoikis. Thus, cancer cell mRNA translation involves two cap-dependent mRNA translation mechanisms, eIF4E/mTORC1 and DAP5/eIF3d. These findings highlight a surprising level of plasticity in mRNA translation during cancer progression and metastasis.
Keywords: CP: Cancer; CP: Molecular biology; DAP5; breast cancer; epithelial-to-mesenchymal transition; metastasis; protein synthesis; translation initiation.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

