Protective effects of genistein on the production performance and lipid metabolism disorders in laying hens with fatty liver hemorrhagic syndrome by activation of the GPER-AMPK signaling pathways
- PMID: 37314978
- PMCID: PMC10290500
- DOI: 10.1093/jas/skad197
Protective effects of genistein on the production performance and lipid metabolism disorders in laying hens with fatty liver hemorrhagic syndrome by activation of the GPER-AMPK signaling pathways
Abstract
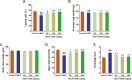
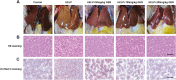
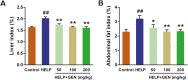
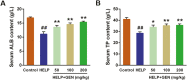
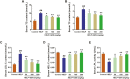
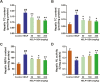
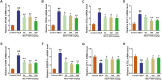
The aim of this study was to evaluate the beneficial effects and potential mechanisms of genistein (GEN) on production performance impairments and lipid metabolism disorders in laying hens fed a high-energy and low-protein (HELP) diet. A total of 120 Hy-line Brown laying hens were fed with the standard diet and HELP diet supplemented with 0, 50, 100, and 200 mg/kg GEN for 80 d. The results showed that the declines in laying rate (P < 0.01), average egg weight (P < 0.01), and egg yield (P < 0.01), and the increase of the ratio of feed to egg (P < 0.01) induced by HELP diet were markedly improved by 100 and 200 mg/kg of GEN treatment in laying hens (P < 0.05). Moreover, the hepatic steatosis and increases of lipid contents (P < 0.01) in serum and liver caused by HELP diet were significantly alleviated by treatment with 100 and 200 mg/kg of GEN in laying hens (P < 0.05). The liver index and abdominal fat index of laying hens in the HELP group were higher than subjects in the control group (P < 0.01), which were evidently attenuated by dietary 50 to 200 mg/kg of GEN supplementation (P < 0.05). Dietary 100 and 200 mg/kg of GEN supplementation significantly reduced the upregulations of genes related to fatty acid transport and synthesis (P < 0.01) but enhanced the downregulations of genes associated with fatty acid oxidation (P < 0.01) caused by HELP in the liver of laying hens (P < 0.05). Importantly, 100 and 200 mg/kg of GEN supplementation markedly increased G protein-coupled estrogen receptor (GPER) mRNA and protein expression levels and activated the AMP-activated protein kinase (AMPK) signaling pathway in the liver of laying hens fed a HELP diet (P < 0.05). These data indicated that the protective effects of GEN against the decline of production performance and lipid metabolism disorders caused by HELP diet in laying hens may be related to the activation of the GPER-AMPK signaling pathways. These data not only provide compelling evidence for the protective effect of GEN against fatty liver hemorrhagic syndrome in laying hens but also provide the theoretical basis for GEN as an additive to alleviate metabolic disorders in poultry.
Keywords: G protein-coupled estrogen receptor–AMP-activated protein kinase signaling pathways; fatty liver hemorrhagic syndrome; genistein; laying hens; lipid metabolism disorders.
Plain language summary
Fatty liver hemorrhagic syndrome (FLHS) is a nutritional and metabolic disease that seriously threatens the health and performance of laying hens, which is characterized by hepatic steatosis and lipid metabolism disorders. As an isoflavone phytoestrogen, genistein (GEN) exerts many beneficial functions, including alleviating lipid metabolism disorders and anti-inflammatory properties. However, further research is needed on the protective effect and potential mechanism of GEN on the FLHS in laying hens. Here, we found that GEN treatment improved liver injury and decline of production performance in laying hens with FLHS. Moreover, GEN treatment alleviated hepatic steatosis and lipid metabolism disorders through reducing the expression levels of mRNA related to fatty acid transport and synthesis and enhancing the mRNA expression levels of factors associated with fatty acid oxidation in FLHS layers, which may be achieved by activation of the G protein-coupled estrogen receptor–adenosine 5'-monophosphate (AMP)-activated protein kinase signaling pathways. These data not only provide compelling evidence for the protective effects and mechanisms of GEN against FLHS in laying hens but also provide the theoretical basis for GEN to alleviate other metabolic disorders in poultry.
© The Author(s) 2023. Published by Oxford University Press on behalf of the American Society of Animal Science. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
The authors declare that there are no competing interests associated with this study.
Figures

Similar articles
-
Dehydroepiandrosterone activates the GPER-mediated AMPK signaling pathway to alleviate the oxidative stress and inflammatory response in laying hens fed with high-energy and low-protein diets.Life Sci. 2022 Nov 1;308:120926. doi: 10.1016/j.lfs.2022.120926. Epub 2022 Sep 1. Life Sci. 2022. PMID: 36058264
-
Dioscin improves fatty liver hemorrhagic syndrome by promoting ERα-AMPK mediated mitophagy in laying hens.Phytomedicine. 2024 Dec;135:156056. doi: 10.1016/j.phymed.2024.156056. Epub 2024 Sep 19. Phytomedicine. 2024. PMID: 39342780
-
Coated cysteamine and choline chloride could be potential feed additives to mitigate the harmful effects of fatty liver hemorrhagic syndrome in laying hens caused by high-energy low-protein diet.Poult Sci. 2024 Dec;103(12):104296. doi: 10.1016/j.psj.2024.104296. Epub 2024 Sep 5. Poult Sci. 2024. PMID: 39305615 Free PMC article.
-
Protective effect of Artemisia capillaris on hepatic lipid accumulation in laying hens with fatty liver and the study of its mechanism.Br Poult Sci. 2025 Jun 9:1-12. doi: 10.1080/00071668.2025.2512585. Online ahead of print. Br Poult Sci. 2025. PMID: 40488218 Review.
-
Mechanisms of hepatic and renal injury in lipid metabolism disorders in metabolic syndrome.Int J Biol Sci. 2024 Sep 9;20(12):4783-4798. doi: 10.7150/ijbs.100394. eCollection 2024. Int J Biol Sci. 2024. PMID: 39309427 Free PMC article. Review.
Cited by
-
Solid Dispersions of Genistein via Solvent Rotary Evaporation for Improving Solubility, Bioavailability, and Amelioration Effect in HFD-Induced Obesity Mice.Pharmaceutics. 2024 Feb 22;16(3):306. doi: 10.3390/pharmaceutics16030306. Pharmaceutics. 2024. PMID: 38543200 Free PMC article.
-
Caffeic acid and chlorogenic acid mediate the ADPN-AMPK-PPARα pathway to improve fatty liver and production performance in laying hens.J Anim Sci Biotechnol. 2025 Apr 3;16(1):49. doi: 10.1186/s40104-025-01175-z. J Anim Sci Biotechnol. 2025. PMID: 40176148 Free PMC article.
-
Bile acid disorders and intestinal barrier dysfunction are involved in the development of fatty liver in laying hens.Poult Sci. 2024 Dec;103(12):104422. doi: 10.1016/j.psj.2024.104422. Epub 2024 Oct 13. Poult Sci. 2024. PMID: 39418789 Free PMC article.
-
Dietary Puerariae Lobatae Radix polysaccharides improve ovarian function and reproductive efficiency in laying hens with fatty liver hemorrhagic syndrome.Poult Sci. 2025 May;104(5):105062. doi: 10.1016/j.psj.2025.105062. Epub 2025 Mar 18. Poult Sci. 2025. PMID: 40120252 Free PMC article.
References
-
- Amanat, S., Eftekhari M. H., Fararouei M., Bagheri Lankarani K., and Massoumi S. J.. . 2018. Genistein supplementation improves insulin resistance and inflammatory state in non-alcoholic fatty liver patients: a randomized, controlled trial. Clin. Nutr. 37:1210–1215. doi:10.1016/j.clnu.2017.05.028. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- 32202758/National Natural Science Foundation of China
- KYQN2023006/Fundamental Research Funds for the Central Universities
- BK20210400/Natural Science Foundation of Jiangsu Province
- 2021M691617/China Postdoctoral Science Foundation
- Priority Academic Program Development of Jiangsu Higher Education Institutions
LinkOut - more resources
Full Text Sources
Medical
Research Materials

