Stochastic simulations of self-organized elastogenesis in the developing lung
- PMID: 37315061
- PMCID: PMC10298752
- DOI: 10.1371/journal.pcbi.1011219
Stochastic simulations of self-organized elastogenesis in the developing lung
Abstract
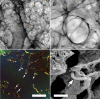
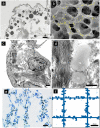
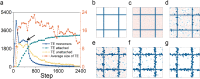
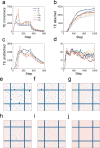
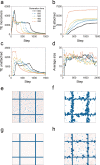
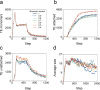
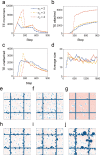
In the normal lung, the dominant cable is an elastic "line element" composed of elastin fibers bound to a protein scaffold. The cable line element maintains alveolar geometry by balancing surface forces within the alveolus and changes in lung volume with exercise. Recent work in the postnatal rat lung has suggested that the process of cable development is self-organized in the extracellular matrix. Early in postnatal development, a blanket of tropoelastin (TE) spheres appear in the primitive lung. Within 7 to 10 days, the TE spheres are incorporated into a distributed protein scaffold creating the mature cable line element. To study the process of extracellular assembly, we used cellular automata (CA) simulations. CA simulations demonstrated that the intermediate step of tropoelastin self-aggregation into TE spheres enhanced the efficiency of cable formation more than 5-fold. Similarly, the rate of tropoelastin production had a direct impact on the efficiency of scaffold binding. The binding affinity of the tropoelastin to the protein scaffold, potentially reflecting heritable traits, also had a significant impact on cable development. In contrast, the spatial distribution of TE monomer production, increased Brownian motion and variations in scaffold geometry did not significantly impact simulations of cable development. We conclude that CA simulations are useful in exploring the impact of concentration, geometry, and movement on the fundamental process of elastogenesis.
Copyright: © 2023 Fan et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Extracellular Assembly of the Elastin Cable Line Element in the Developing Lung.Anat Rec (Hoboken). 2017 Sep;300(9):1670-1679. doi: 10.1002/ar.23603. Epub 2017 Apr 17. Anat Rec (Hoboken). 2017. PMID: 28380679 Free PMC article.
-
Elastic fiber ultrastructure and assembly.Matrix Biol. 2019 Nov;84:31-40. doi: 10.1016/j.matbio.2019.10.002. Epub 2019 Oct 24. Matrix Biol. 2019. PMID: 31669522 Free PMC article. Review.
-
Oxidative and nitrosative modifications of tropoelastin prevent elastic fiber assembly in vitro.J Biol Chem. 2010 Nov 26;285(48):37396-404. doi: 10.1074/jbc.M110.126789. Epub 2010 Sep 16. J Biol Chem. 2010. PMID: 20847053 Free PMC article.
-
Exposure of tropoelastin to peroxynitrous acid gives high yields of nitrated tyrosine residues, di-tyrosine cross-links and altered protein structure and function.Free Radic Biol Med. 2018 Feb 1;115:219-231. doi: 10.1016/j.freeradbiomed.2017.11.019. Epub 2017 Nov 27. Free Radic Biol Med. 2018. PMID: 29191462
-
Tropoelastin.Int J Biochem Cell Biol. 2009 Mar;41(3):494-7. doi: 10.1016/j.biocel.2008.03.017. Epub 2008 Apr 1. Int J Biochem Cell Biol. 2009. PMID: 18468477 Review.
References
-
- Weibel ER. Commentaries on viewpoint: Unresolved mysteries change the perspective! J Appl Physiol. 2012;113(12):1948-. WOS:000312499900016. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

