Impact of statin withdrawal on perceived and objective muscle function
- PMID: 37315062
- PMCID: PMC10266600
- DOI: 10.1371/journal.pone.0281178
Impact of statin withdrawal on perceived and objective muscle function
Abstract
Background and aims: Statin-associated muscle symptoms (SAMS) are frequently reported. Nevertheless, few data on objective measures of muscle function are available. Recent data suggesting an important nocebo effect with statin use could confound such effects. The objective was to assess if subjective and objective measures of muscle function improve after drug withdrawal in SAMS reporters.
Methods: Patients (59 men, 33 women, 50.3±9.6 yrs.) in primary cardiovascular prevention composed three cohorts: statin users with (SAMS, n = 61) or without symptoms (No SAMS, n = 15), and controls (n = 16) (registered at clinicaltrials.gov, NCT01493648). Force (F), endurance (E) and power (P) of the leg extensors (ext) and flexors (fle) and handgrip strength (Fhg) were measured using isokinetic and handheld dynamometers, respectively. A 10-point visual analogue scale (VAS) was used to self-assess SAMS intensity. Measures were taken before and after two months of withdrawal.
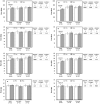
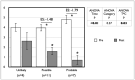
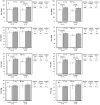
Results: Following withdrawal, repeated-measures analyses show improvements for the entire cohort in Eext, Efle, Ffle, Pext and Pfle (range +7.2 to +13.3%, all p≤0.02). Post-hoc analyses show these changes to occur notably in SAMS (+8.8 to +16.6%), concurrent with a decrease in subjective perception of effects in SAMS (VAS, from 5.09 to 1.85). Fhg was also improved in SAMS (+4.0 to +6.2%) when compared to No SAMS (-1.7 to -4.2%) (all p = 0.02).
Conclusions: Whether suffering from "true" SAMS or nocebo, those who reported SAMS had modest but relevant improvements in muscle function concurrent with a decrease in subjective symptoms intensity after drug withdrawal. Greater attention by clinicians to muscle function in frail statin users appears warranted.
Trial registration: This study is registered in clinicaltrials.gov (NCT01493648).
Copyright: © 2023 Peyrel et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al.. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082–e143. doi: 10.1161/CIR.0000000000000625 - DOI - PMC - PubMed
-
- Boulanger-Piette A, Bergeron J, Desgreniers J, Côté-Levesque M, Brassard D, Joanisse DR, et al.. [Statin intolerance and associated muscular dysfunctions]. Med Sci (Paris). 2015;31(12):1109–14. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

