Structural and mechanistic basis of neutralization by a pan-hantavirus protective antibody
- PMID: 37315110
- PMCID: PMC11721787
- DOI: 10.1126/scitranslmed.adg1855
Structural and mechanistic basis of neutralization by a pan-hantavirus protective antibody
Abstract
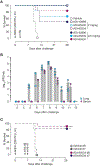
Emerging rodent-borne hantaviruses cause severe diseases in humans with no approved vaccines or therapeutics. We recently isolated a monoclonal broadly neutralizing antibody (nAb) from a Puumala virus-experienced human donor. Here, we report its structure bound to its target, the Gn/Gc glycoprotein heterodimer comprising the viral fusion complex. The structure explains the broad activity of the nAb: It recognizes conserved Gc fusion loop sequences and the main chain of variable Gn sequences, thereby straddling the Gn/Gc heterodimer and locking it in its prefusion conformation. We show that the nAb's accelerated dissociation from the divergent Andes virus Gn/Gc at endosomal acidic pH limits its potency against this highly lethal virus and correct this liability by engineering an optimized variant that sets a benchmark as a candidate pan-hantavirus therapeutic.
Conflict of interest statement
Figures



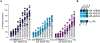
References
-
- Watson DC, Sargianou M, Papa A, Chra P, Starakis I, Panos G, Epidemiology of Hantavirus infections in humans: A comprehensive, global overview. Crit. Rev. Microbiol 40, 261–272 (2014). - PubMed
-
- Martínez VP, Di Paola N, Alonso DO, Pérez-Sautu U, Bellomo CM, Iglesias AA, Coelho RM, López B, Periolo N, Larson PA, Nagle ER, Chitty JA, Pratt CB, Díaz J, Cisterna D, Campos J, Sharma H, Dighero-Kemp B, Biondo E, Lewis L, Anselmo C, Olivera CP, Pontoriero F, Lavarra E, Kuhn JH, Strella T, Edelstein A, Burgos MI, Kaler M, Rubinstein A, Kugelman JR, Sanchez-Lockhart M, Perandones C, Palacios G, “Super-Spreaders” and Person-to-Person Transmission of Andes Virus in Argentina. N. Engl. J. Med 383, 2230–2241 (2020). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

