CD62L expression marks SARS-CoV-2 memory B cell subset with preference for neutralizing epitopes
- PMID: 37315144
- PMCID: PMC10266721
- DOI: 10.1126/sciadv.adf0661
CD62L expression marks SARS-CoV-2 memory B cell subset with preference for neutralizing epitopes
Abstract
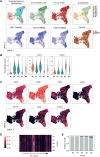
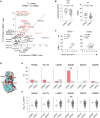
Severe acute respiratory syndrome coronavirus 2-neutralizing antibodies primarily target the spike receptor binding domain (RBD). However, B cell antigen receptors (BCRs) on RBD-binding memory B (Bmem) cells have variation in the neutralizing activities. Here, by combining single Bmem cell profiling with antibody functional assessment, we dissected the phenotype of Bmem cell harboring the potently neutralizing antibodies in coronavirus disease 2019 (COVID-19)-convalescent individuals. The neutralizing subset was marked by an elevated CD62L expression and characterized by distinct epitope preference and usage of convergent VH (variable region of immunoglobulin heavy chain) genes, accounting for the neutralizing activities. Concordantly, the correlation was observed between neutralizing antibody titers in blood and CD62L+ subset, despite the equivalent RBD binding of CD62L+ and CD62L- subset. Furthermore, the kinetics of CD62L+ subset differed between the patients who recovered from different COVID-19 severities. Our Bmem cell profiling reveals the unique phenotype of Bmem cell subset that harbors potently neutralizing BCRs, advancing our understanding of humoral protection.
Figures



References
-
- T. Kurosaki, K. Kometani, W. Ise, Memory B cells. Nat. Rev. Immunol. 15, 149–159 (2015). - PubMed
-
- R. R. Goel, M. M. Painter, S. A. Apostolidis, D. Mathew, W. Meng, A. M. Rosenfeld, K. A. Lundgreen, A. Reynaldi, D. S. Khoury, A. Pattekar, S. Gouma, L. Kuri-Cervantes, P. Hicks, S. Dysinger, A. Hicks, H. Sharma, S. Herring, S. Korte, A. E. Baxter, D. A. Oldridge, J. R. Giles, M. E. Weirick, C. M. McAllister, M. Awofolaju, N. Tanenbaum, E. M. Drapeau, J. Dougherty, S. Long, K. D'Andrea, J. T. Hamilton, M. McLaughlin, J. C. Williams, S. Adamski, O. Kuthuru; UPenn COVID Processing Unit, I. Frank, M. R. Betts, L. A. Vella, A. Grifoni, D. Weiskopf, A. Sette, S. E. Hensley, M. P. Davenport, P. Bates, E. T. L. Prak, A. R. Greenplate, E. J. Wherry, mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science 374, abm0829 (2021). - PMC - PubMed
-
- D. S. Khoury, D. Cromer, A. Reynaldi, T. E. Schlub, A. K. Wheatley, J. A. Juno, K. Subbarao, S. J. Kent, J. A. Triccas, M. P. Davenport, Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 27, 1205–1211 (2021). - PubMed
-
- E. Andreano, E. Nicastri, I. Paciello, P. Pileri, N. Manganaro, G. Piccini, A. Manenti, E. Pantano, A. Kabanova, M. Troisi, F. Vacca, D. Cardamone, C. De Santi, J. L. Torres, G. Ozorowski, L. Benincasa, H. Jang, C. Di Genova, L. Depau, J. Brunetti, C. Agrati, M. R. Capobianchi, C. Castilletti, A. Emiliozzi, M. Fabbiani, F. Montagnani, L. Bracci, G. Sautto, T. M. Ross, E. Montomoli, N. Temperton, A. B. Ward, C. Sala, G. Ippolito, R. Rappuoli, Extremely potent human monoclonal antibodies from COVID-19 convalescent patients. Cell 184, 1821–1835.e16 (2021). - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

