Exploring alternative pathways for the in vitro establishment of the HOPAC cycle for synthetic CO2 fixation
- PMID: 37315145
- PMCID: PMC10266731
- DOI: 10.1126/sciadv.adh4299
Exploring alternative pathways for the in vitro establishment of the HOPAC cycle for synthetic CO2 fixation
Abstract
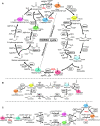
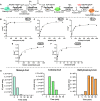
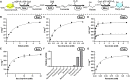
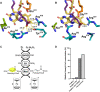
Nature has evolved eight different pathways for the capture and conversion of CO2, including the Calvin-Benson-Bassham cycle of photosynthesis. Yet, these pathways underlie constrains and only represent a fraction of the thousands of theoretically possible solutions. To overcome the limitations of natural evolution, we introduce the HydrOxyPropionyl-CoA/Acrylyl-CoA (HOPAC) cycle, a new-to-nature CO2-fixation pathway that was designed through metabolic retrosynthesis around the reductive carboxylation of acrylyl-CoA, a highly efficient principle of CO2 fixation. We realized the HOPAC cycle in a step-wise fashion and used rational engineering approaches and machine learning-guided workflows to further optimize its output by more than one order of magnitude. Version 4.0 of the HOPAC cycle encompasses 11 enzymes from six different organisms, converting ~3.0 mM CO2 into glycolate within 2 hours. Our work moves the hypothetical HOPAC cycle from a theoretical design into an established in vitro system that forms the basis for different potential applications.
Figures



Similar articles
-
Creating new-to-nature carbon fixation: A guide.Metab Eng. 2024 Mar;82:12-28. doi: 10.1016/j.ymben.2023.12.012. Epub 2023 Dec 29. Metab Eng. 2024. PMID: 38160747 Review.
-
Augmenting the Calvin-Benson-Bassham cycle by a synthetic malyl-CoA-glycerate carbon fixation pathway.Nat Commun. 2018 May 22;9(1):2008. doi: 10.1038/s41467-018-04417-z. Nat Commun. 2018. PMID: 29789614 Free PMC article.
-
An engineered Calvin-Benson-Bassham cycle for carbon dioxide fixation in Methylobacterium extorquens AM1.Metab Eng. 2018 May;47:423-433. doi: 10.1016/j.ymben.2018.04.003. Epub 2018 Apr 4. Metab Eng. 2018. PMID: 29625224
-
A synthetic pathway for the fixation of carbon dioxide in vitro.Science. 2016 Nov 18;354(6314):900-904. doi: 10.1126/science.aah5237. Science. 2016. PMID: 27856910 Free PMC article.
-
Yeast metabolic engineering for carbon dioxide fixation and its application.Bioresour Technol. 2022 Feb;346:126349. doi: 10.1016/j.biortech.2021.126349. Epub 2021 Nov 17. Bioresour Technol. 2022. PMID: 34800639 Review.
Cited by
-
Perspectives for Using CO2 as a Feedstock for Biomanufacturing of Fuels and Chemicals.Bioengineering (Basel). 2023 Nov 26;10(12):1357. doi: 10.3390/bioengineering10121357. Bioengineering (Basel). 2023. PMID: 38135948 Free PMC article. Review.
-
Computation-aided designs enable developing auxotrophic metabolic sensors for wide-range glyoxylate and glycolate detection.Nat Commun. 2025 Mar 4;16(1):2168. doi: 10.1038/s41467-025-57407-3. Nat Commun. 2025. PMID: 40038270 Free PMC article.
-
Multi-scale reactor designs extend the physical limits of fixation.bioRxiv [Preprint]. 2024 Aug 30:2024.08.28.610213. doi: 10.1101/2024.08.28.610213. bioRxiv. 2024. PMID: 39257791 Free PMC article. Preprint.
-
MACAW: a method for semi-automatic detection of errors in genome-scale metabolic models.Genome Biol. 2025 Mar 28;26(1):79. doi: 10.1186/s13059-025-03533-6. Genome Biol. 2025. PMID: 40156030 Free PMC article.
-
Quantitative Online Monitoring of an Immobilized Enzymatic Network by Ion Mobility-Mass Spectrometry.J Am Chem Soc. 2024 Jul 31;146(30):20778-20787. doi: 10.1021/jacs.4c04218. Epub 2024 Jul 16. J Am Chem Soc. 2024. PMID: 39013149 Free PMC article.
References
-
- Wurtzel E. T., Vickers C., Hanson A. D., Millar A. H., Cooper M., Voss-Fels K. P., Nikel P. I., Erb T. J., Revolutionizing agriculture with synthetic biology. Nat. Plants 5, 1207–1210 (2019). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

