First Pharmacokinetic Data of Tenofovir Alafenamide Fumarate and Tenofovir With Dolutegravir or Boosted Protease Inhibitors in African Children: A Substudy of the CHAPAS-4 Trial
- PMID: 37315296
- PMCID: PMC10506774
- DOI: 10.1093/cid/ciad267
First Pharmacokinetic Data of Tenofovir Alafenamide Fumarate and Tenofovir With Dolutegravir or Boosted Protease Inhibitors in African Children: A Substudy of the CHAPAS-4 Trial
Abstract
Background: We evaluated the pharmacokinetics of tenofovir alafenamide fumarate (TAF) and tenofovir in a subset of African children enrolled in the CHAPAS-4 trial.
Methods: Children aged 3-15 years with human immunodeficiency virus infection failing first-line antiretroviral therapy were randomized to emtricitabine/TAF versus standard-of-care nucleoside reverse transcriptase inhibitor combination, plus dolutegravir, atazanavir/ritonavir, darunavir/ritonavir, or lopinavir/ritonavir. Daily emtricitabine/TAF was dosed according to World Health Organization (WHO)-recommended weight bands: 120/15 mg in children weighing 14 to <25 kg and 200/25 mg in those weighing ≥25 kg. At steady state, 8-9 blood samples were taken to construct pharmacokinetic curves. Geometric mean (GM) area under the concentration-time curve (AUC) and the maximum concentration (Cmax) were calculated for TAF and tenofovir and compared to reference exposures in adults.
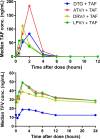
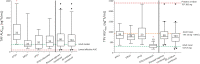
Results: Pharmacokinetic results from 104 children taking TAF were analyzed. GM (coefficient of variation [CV%]) TAF AUClast when combined with dolutegravir (n = 18), darunavir/ritonavir (n = 34), or lopinavir/ritonavir (n = 20) were 284.5 (79), 232.0 (61), and 210.2 (98) ng*hour/mL, respectively, and were comparable to adult reference values. When combined with atazanavir/ritonavir (n = 32), TAF AUClast increased to 511.4 (68) ng*hour/mL. For each combination, tenofovir GM (CV%) AUCtau and Cmax remained below reference values in adults taking 25 mg TAF with a boosted protease inhibitors.
Conclusions: In children, TAF combined with boosted PIs or dolutegravir and dosed according to WHO-recommended weight bands provides TAF and tenofovir concentrations previously demonstrated to be well tolerated and effective in adults. These data provide the first evidence for use of these combinations in African children.
Clinical trials registration: ISRCTN22964075.
Keywords: HIV; TAF; children; drug interaction; pharmacokinetics.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. D. M. B. has received research grants from ViiV Healthcare, Merck, and Gilead Sciences; payments from ViiV Healthcare and Gilead Sciences for serving on advisory boards; payment from ViiV Healthcare for speaking at symposia; payment or honoraria for lectures from Pfizer and Gilead Sciences and for advisory board for Merck; and is the co-founder of Global DDI Solutions. A. C. has received honoraria from Merck Sharp & Dohme and Gilead (fees paid to institution) and has received study grants from MSD, Gilead Sciences, and ViiV Healthcare. V. Mus. reports honoraria for speaking at conference/webinar from ViiV Healthcare; support to attend international conference from Viatris; and membership on a data and safety monitoring board and participation in advisory board meetings with ViiV Healthcare. A. B. reports a paid consultancy in relation to treatment of COVID-19 in children, completed April 2022, from Gilead. C. C. reports grants or contracts from the EDCTP. V. Mul. reports a role as a committee member of the Technical Committee of Pharmacovigilance and Clinical Trials of the Zambia Medicines Regulatory Authority, with attendance allowance paid to author; and receipt of donated drugs for the main CHAPAS-4 Trial from Janssen, Emcure, Cipla, and Gilead Sciences. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
-
- World Health Organization . Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. 2021:548. Available at: https://www.who.int/publications/i/item/9789240031593. Accessed 29 September 2022.
-
- Ruane PJ, DeJesus E, Berger D, et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of tenofovir alafenamide as 10-day monotherapy in HIV-1–positive adults. J Acquir Immune Defic Syndr 2013; 63:449–55. - PubMed
-
- US Food and Drug Administration . Descovy—clinical pharmacology and biopharmaceutics review(s). 2015. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/208215Orig1s000C.... Accessed 15 November 2022.
-
- European Medicines Agency . Descovy—summary of product characteristics. 2021. Available at: https://www.ema.europa.eu/en/documents/product-information/descovy-epar-.... Accessed 15 November 2022.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

