Divergent structures of Mammalian and gammaherpesvirus uracil DNA glycosylases confer distinct DNA binding and substrate activity
- PMID: 37315375
- PMCID: PMC10441670
- DOI: 10.1016/j.dnarep.2023.103515
Divergent structures of Mammalian and gammaherpesvirus uracil DNA glycosylases confer distinct DNA binding and substrate activity
Abstract
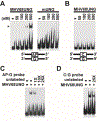
Uracil DNA glycosylase (UNG) removes mutagenic uracil base from DNA to initiate base excision repair (BER). The result is an abasic site (AP site) that is further processed by the high-fidelity BER pathway to complete repair and maintain genome integrity. The gammaherpesviruses (GHVs), human Kaposi sarcoma herpesvirus (KSHV), Epstein-Barr virus (EBV), and murine gammaherpesvirus 68 (MHV68) encode functional UNGs that have a role in viral genome replication. Mammalian and GHVs UNG share overall structure and sequence similarity except for a divergent amino-terminal domain and a leucine loop motif in the DNA binding domain that varies in sequence and length. To determine if divergent domains contribute to functional differences between GHV and mammalian UNGs, we analyzed their roles in DNA interaction and catalysis. By utilizing chimeric UNGs with swapped domains we found that the leucine loop in GHV, but not mammalian UNGs facilitates interaction with AP sites and that the amino-terminal domain modulates this interaction. We also found that the leucine loop structure contributes to differential UDGase activity on uracil in single- versus double-stranded DNA. Taken together we demonstrate that the GHV UNGs evolved divergent domains from their mammalian counterparts that contribute to differential biochemical properties from their mammalian counterparts.
Keywords: Abasic site; Gammaherpesvirus; Uracil DNA glycosylase.
Copyright © 2023 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


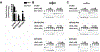


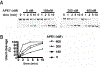
Similar articles
-
A structurally conserved motif in γ-herpesvirus uracil-DNA glycosylases elicits duplex nucleotide-flipping.Nucleic Acids Res. 2018 May 4;46(8):4286-4300. doi: 10.1093/nar/gky217. Nucleic Acids Res. 2018. PMID: 29596604 Free PMC article.
-
Upregulation of Uracil DNA Glycosylase (UNG) in Prostate Cancer.Cancer Genet. 2025 Sep;296-297:130-132. doi: 10.1016/j.cancergen.2025.07.005. Epub 2025 Jul 7. Cancer Genet. 2025. PMID: 40652631
-
New insights on the role of the gamma-herpesvirus uracil-DNA glycosylase leucine loop revealed by the structure of the Epstein-Barr virus enzyme in complex with an inhibitor protein.J Mol Biol. 2007 Feb 9;366(1):117-31. doi: 10.1016/j.jmb.2006.11.007. Epub 2006 Nov 7. J Mol Biol. 2007. PMID: 17157317
-
The intricate structural chemistry of base excision repair machinery: implications for DNA damage recognition, removal, and repair.DNA Repair (Amst). 2007 Apr 1;6(4):410-28. doi: 10.1016/j.dnarep.2006.10.004. Epub 2007 Jan 8. DNA Repair (Amst). 2007. PMID: 17208522 Review.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
Cited by
-
Sequencing of Kaposi's Sarcoma Herpesvirus (KSHV) genomes from persons of diverse ethnicities and provenances with KSHV-associated diseases demonstrate multiple infections, novel polymorphisms, and low intra-host variance.PLoS Pathog. 2024 Jul 15;20(7):e1012338. doi: 10.1371/journal.ppat.1012338. eCollection 2024 Jul. PLoS Pathog. 2024. PMID: 39008527 Free PMC article.
-
Kaposi Sarcoma-Associated Herpesvirus Sequencing in People Living With HIV in the Southern United States Reveals Subtype Diversity and Multiple Infections.J Med Virol. 2025 Jul;97(7):e70467. doi: 10.1002/jmv.70467. J Med Virol. 2025. PMID: 40616589 Free PMC article.
-
Uracil-DNA glycosylase of murine gammaherpesvirus 68 binds cognate viral replication factors independently of its catalytic residues.mSphere. 2023 Oct 24;8(5):e0027823. doi: 10.1128/msphere.00278-23. Epub 2023 Sep 25. mSphere. 2023. PMID: 37747202 Free PMC article.
References
-
- Krokan HE, Saetrom P, Aas PA, Pettersen HS, Kavli B, Slupphaug G, Error-free versus mutagenic processing of genomic uracil--relevance to cancer, DNA Repair (Amst), 19 (2014) 38–47. - PubMed
-
- Safavi S, Larouche A, Zahn A, Patenaude AM, Domanska D, Dionne K, Rognes T, Dingler F, Kang SK, Liu Y, Johnson N, Hebert J, Verdun RE, Rada CA, Vega F, Nilsen H, Di Noia JM, The uracil-DNA glycosylase UNG protects the fitness of normal and cancer B cells expressing AID, NAR Cancer, 2 (2020) zcaa019. - PMC - PubMed
-
- Di Noia JM, Neuberger MS, Molecular mechanisms of antibody somatic hypermutation, Annu Rev Biochem, 76 (2007) 1–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

