Ultra-high dose-rate proton FLASH improves tumor control
- PMID: 37315577
- PMCID: PMC10527231
- DOI: 10.1016/j.radonc.2023.109741
Ultra-high dose-rate proton FLASH improves tumor control
Abstract
Background and purpose: Proton radiotherapy (PRT) offers potential benefits over other radiation modalities, including photon and electron radiotherapy. Increasing the rate at which proton radiation is delivered may provide a therapeutic advantage. Here, we compared the efficacy of conventional proton therapy (CONVpr) to ultrahigh dose-rate proton therapy, FLASHpr, in a mouse model of non-small cell lung cancers (NSCLC).
Materials and methods: Mice bearing orthotopic lung tumors received thoracic radiation therapy using CONVpr (<0.05 Gy/s) and FLASHpr (>60 Gy/s) dose rates.
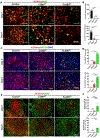
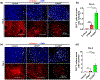
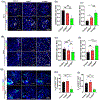
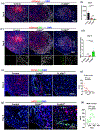
Results: Compared to CONVpr, FLASHpr was more effective in reducing tumor burden and decreasing tumor cell proliferation. Furthermore, FLASHpr was more efficient in increasing the infiltration of cytotoxic CD8+ T-lymphocytes inside the tumor while simultaneously reducing the percentage of immunosuppressive regulatory T-cells (Tregs) among T-lymphocytes. Also, compared to CONVpr, FLASHpr was more effective in decreasing pro-tumorigenic M2-like macrophages in lung tumors, while increasing infiltration of anti-tumor M1-like macrophages. Finally, FLASHpr treatment reduced expression of checkpoint inhibitors in lung tumors, indicating reduced immune tolerance.
Conclusions: Our results suggest that FLASH dose-rate proton delivery modulates the immune system to improve tumor control and might thus be a promising new alternative to conventional dose rates for NSCLC treatment.
Keywords: FLASH; Lung cancer; Mouse model; Proton radiotherapy; Ultrahigh dose-rate proton.
Copyright © 2023 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Graves PR, Siddiqui F, Anscher MS, Movsas B. Radiation pulmonary toxicity: from mechanisms to management. Semin Radiat Oncol. 2010;20(3):201–207. - PubMed
-
- Ramella S, D'Angelillo RM. Proton beam or photon beam radiotherapy in the treatment of non-small-cell lung cancer. Lancet Oncol. 2020;21(7):873–875. - PubMed
-
- Stauder MC, Macdonald OK, Olivier KR, et al. Early pulmonary toxicity following lung stereotactic body radiation therapy delivered in consecutive daily fractions. Radiother Oncol. 2011;99(2):166–171. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

