A combined DHA-rich fish oil and cocoa flavanols intervention does not improve cognition or brain structure in older adults with memory complaints: results from the CANN randomized, controlled parallel-design study
- PMID: 37315924
- PMCID: PMC10447509
- DOI: 10.1016/j.ajcnut.2023.06.008
A combined DHA-rich fish oil and cocoa flavanols intervention does not improve cognition or brain structure in older adults with memory complaints: results from the CANN randomized, controlled parallel-design study
Abstract
Background: There is evidence that both omega-3 long-chain polyunsaturated fatty acids (PUFAs) (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) and cocoa flavanols can improve cognitive performance in both healthy individuals and in those with memory complaints. However, their combined effect is unknown.
Objectives: To investigate the combined effect of EPA/DHA and cocoa flavanols (OM3FLAV) on cognitive performance and brain structures in older adults with memory complaints.
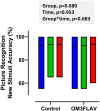
Methods: A randomized placebo-controlled trial of DHA-rich fish oil (providing 1.1 g/d DHA and 0.4 g/d EPA) and a flavanol-rich dark chocolate (providing 500 mg/d flavan-3-ols) was conducted in 259 older adults with either subjective cognitive impairment or mild cognitive impairment. Participants underwent assessment at baseline, 3 mo, and 12 mo. The primary outcome was the number of false-positives on a picture recognition task from the Cognitive Drug Research computerized assessment battery. Secondary outcomes included other cognition and mood outcomes, plasma lipids, brain-derived neurotrophic factor (BDNF), and glucose levels. A subset of 110 participants underwent structural neuroimaging at baseline and at 12 mo.
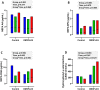
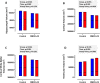
Results: 197 participants completed the study. The combined intervention had no significant effect on any cognitive outcomes, with the exception of reaction time variability (P = 0.007), alertness (P < 0.001), and executive function (P < 0.001), with a decline in function observed in the OM3FLAV group (118.6 [SD 25.3] at baseline versus 113.3 [SD 25.4] at 12 mo for executive function) relative to the control, and an associated decrease in cortical volume (P = 0.039). Compared with the control group, OM3FLAV increased plasma HDL, total cholesterol ratio (P < 0.001), and glucose (P = 0.008) and reduced TG concentrations (P < 0.001) by 3 mo, which were sustained to 12 mo, with no effect on BDNF. Changes in plasma EPA and DHA and urinary flavonoid metabolite concentrations confirmed compliance to the intervention.
Conclusions: These results suggest that cosupplementation with ω-3 PUFAs and cocoa flavanols for 12 mo does not improve cognitive outcomes in those with cognitive impairment. This trial was registered at clinicaltrials.gov as NCT02525198.
Keywords: aging; cognition; flavonoids; mild cognitive impairment; omega-3; polyphenols; subjective cognitive impairment.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures

Comment in
-
In randomized controlled trials with nutrient supplementation, participants with subjective memory complaints should not be combined with those having a mild cognitive impairment diagnosis.Am J Clin Nutr. 2023 Dec;118(6):1235-1236. doi: 10.1016/j.ajcnut.2023.08.027. Epub 2023 Oct 20. Am J Clin Nutr. 2023. PMID: 37867132 No abstract available.
References
-
- Lewis F., Schaffer S.K., Sussex J., O’Neill P., Cockcroft L. Office of Health Economics (OHE) Contract Research; 2014. The Trajectory of Dementia in the UK – Making a Difference.https://www.ohe.org/publications/trajectory-dementia-uk-making-difference/ [Internet] Available from:
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

