Dimethyl fumarate and 4-octyl itaconate are anticoagulants that suppress Tissue Factor in macrophages via inhibition of Type I Interferon
- PMID: 37316487
- PMCID: PMC10265568
- DOI: 10.1038/s41467-023-39174-1
Dimethyl fumarate and 4-octyl itaconate are anticoagulants that suppress Tissue Factor in macrophages via inhibition of Type I Interferon
Erratum in
-
Publisher Correction: Dimethyl fumarate and 4-octyl itaconate are anticoagulants that suppress Tissue Factor in macrophages via inhibition of Type I Interferon.Nat Commun. 2023 Jul 20;14(1):4374. doi: 10.1038/s41467-023-40034-1. Nat Commun. 2023. PMID: 37474527 Free PMC article. No abstract available.
Abstract
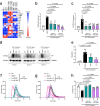
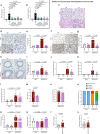
Excessive inflammation-associated coagulation is a feature of infectious diseases, occurring in such conditions as bacterial sepsis and COVID-19. It can lead to disseminated intravascular coagulation, one of the leading causes of mortality worldwide. Recently, type I interferon (IFN) signaling has been shown to be required for tissue factor (TF; gene name F3) release from macrophages, a critical initiator of coagulation, providing an important mechanistic link between innate immunity and coagulation. The mechanism of release involves type I IFN-induced caspase-11 which promotes macrophage pyroptosis. Here we find that F3 is a type I IFN-stimulated gene. Furthermore, F3 induction by lipopolysaccharide (LPS) is inhibited by the anti-inflammatory agents dimethyl fumarate (DMF) and 4-octyl itaconate (4-OI). Mechanistically, inhibition of F3 by DMF and 4-OI involves suppression of Ifnb1 expression. Additionally, they block type I IFN- and caspase-11-mediated macrophage pyroptosis, and subsequent TF release. Thereby, DMF and 4-OI inhibit TF-dependent thrombin generation. In vivo, DMF and 4-OI suppress TF-dependent thrombin generation, pulmonary thromboinflammation, and lethality induced by LPS, E. coli, and S. aureus, with 4-OI additionally attenuating inflammation-associated coagulation in a model of SARS-CoV-2 infection. Our results identify the clinically approved drug DMF and the pre-clinical tool compound 4-OI as anticoagulants that inhibit TF-mediated coagulopathy via inhibition of the macrophage type I IFN-TF axis.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Delvaeye M, Conway EM. Coagulation and innate immune responses: can we view them separately? Blood. 2009;114:2367–2374. - PubMed
-
- Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 2013;13:34–45. - PubMed
-
- Reinhart K, et al. Recognizing sepsis as a global health priority - a WHO resolution. N. Engl. J. Med. 2017;377:414–417. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

