Genome editing of a rice CDP-DAG synthase confers multipathogen resistance
- PMID: 37316672
- PMCID: PMC11575942
- DOI: 10.1038/s41586-023-06205-2
Genome editing of a rice CDP-DAG synthase confers multipathogen resistance
Abstract
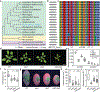
The discovery and application of genome editing introduced a new era of plant breeding by giving researchers efficient tools for the precise engineering of crop genomes1. Here we demonstrate the power of genome editing for engineering broad-spectrum disease resistance in rice (Oryza sativa). We first isolated a lesion mimic mutant (LMM) from a mutagenized rice population. We then demonstrated that a 29-base-pair deletion in a gene we named RESISTANCE TO BLAST1 (RBL1) caused broad-spectrum disease resistance and showed that this mutation caused an approximately 20-fold reduction in yield. RBL1 encodes a cytidine diphosphate diacylglycerol synthase that is required for phospholipid biosynthesis2. Mutation of RBL1 results in reduced levels of phosphatidylinositol and its derivative phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2). In rice, PtdIns(4,5)P2 is enriched in cellular structures that are specifically associated with effector secretion and fungal infection, suggesting that it has a role as a disease-susceptibility factor3. By using targeted genome editing, we obtained an allele of RBL1, named RBL1Δ12, which confers broad-spectrum disease resistance but does not decrease yield in a model rice variety, as assessed in small-scale field trials. Our study has demonstrated the benefits of editing an LMM gene, a strategy relevant to diverse LMM genes and crops.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Figures

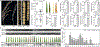
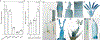
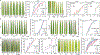








Comment in
-
CRISPR and the Plant Pathologists' Holy Grail.CRISPR J. 2023 Aug;6(4):308-309. doi: 10.1089/crispr.2023.29165.mwi. CRISPR J. 2023. PMID: 37594267 No abstract available.
-
Editing a rice CDP-DAG synthase confers broad-spectrum resistance.Trends Plant Sci. 2023 Dec;28(12):1344-1346. doi: 10.1016/j.tplants.2023.08.011. Epub 2023 Aug 28. Trends Plant Sci. 2023. PMID: 37648632
References
-
- Gao C Genome engineering for crop improvement and future agriculture. Cell 184, 1621–1635 (2021). - PubMed
-
- Zhu H, Li C & Gao C Applications of CRISPR–Cas in agriculture and plant biotechnology. Nat. Rev. Mol. Cell Biol. 21, 661–677 (2020). - PubMed
-
- Chen K, Wang Y, Zhang R, Zhang H & Gao C CRISPR/Cas genome editing and precision plant breeding in agriculture. Annu. Rev. Plant Biol. 70, 667–697 (2019). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

