The neuroscience of cancer
- PMID: 37316719
- PMCID: PMC11146751
- DOI: 10.1038/s41586-023-05968-y
The neuroscience of cancer
Abstract
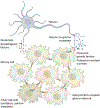
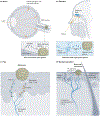
The nervous system regulates tissue stem and precursor populations throughout life. Parallel to roles in development, the nervous system is emerging as a critical regulator of cancer, from oncogenesis to malignant growth and metastatic spread. Various preclinical models in a range of malignancies have demonstrated that nervous system activity can control cancer initiation and powerfully influence cancer progression and metastasis. Just as the nervous system can regulate cancer progression, cancer also remodels and hijacks nervous system structure and function. Interactions between the nervous system and cancer occur both in the local tumour microenvironment and systemically. Neurons and glial cells communicate directly with malignant cells in the tumour microenvironment through paracrine factors and, in some cases, through neuron-to-cancer cell synapses. Additionally, indirect interactions occur at a distance through circulating signals and through influences on immune cell trafficking and function. Such cross-talk among the nervous system, immune system and cancer-both systemically and in the local tumour microenvironment-regulates pro-tumour inflammation and anti-cancer immunity. Elucidating the neuroscience of cancer, which calls for interdisciplinary collaboration among the fields of neuroscience, developmental biology, immunology and cancer biology, may advance effective therapies for many of the most difficult to treat malignancies.
© 2023. Springer Nature Limited.
Conflict of interest statement
Figures
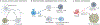
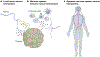

References
-
- Mauch DH et al. CNS synaptogenesis promoted by glia-derived cholesterol. Science 294, 1354–1357 (2001). - PubMed
-
- Ullian EM, Sapperstein SK, Christopherson KS & Barres BA Control of synapse number by glia. Science 291, 657–661 (2001). - PubMed
-
- Christopherson KS et al. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 120, 421–433 (2005). - PubMed
-
- Leclerc C et al. L-type calcium channel activation controls the in vivo transduction of the neuralizing signal in the amphibian embryos. Mech. Dev 64, 105–110 (1997). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

