Metal-enriched HSP90 nanoinhibitor overcomes heat resistance in hyperthermic intraperitoneal chemotherapy used for peritoneal metastases
- PMID: 37316830
- PMCID: PMC10265871
- DOI: 10.1186/s12943-023-01790-2
Metal-enriched HSP90 nanoinhibitor overcomes heat resistance in hyperthermic intraperitoneal chemotherapy used for peritoneal metastases
Abstract
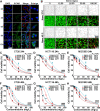
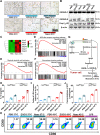
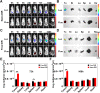
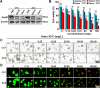
Clinical hyperthermic intraperitoneal chemotherapy (HIPEC) is regarded as a potential treatment that can prolong survival of patients with peritoneal metastases after cytoreductive surgery. However, treated tumor cells are prone to becoming heat resistant to HIPEC therapy through high expression of heat shock proteins (HSPs). Here, a carrier-free bifunctional nanoinhibitor was developed for HIPEC therapy in the management of peritoneal metastases. Self-assembly of the nanoinhibitor was formed by mixing Mn ion and epigallocatechin gallate (EGCG) in a controllable manner. Such nanoinhibitor directly inhibited HSP90 and impaired the HSP90 chaperone cycle by reduced intracellular ATP level. Additionally, heat and Mn ion synergistically induced oxidative stress and expression of caspase 1, which activated GSDMD by proteolysis and caused pyroptosis in tumor cells, triggering immunogenic inflammatory cell death and induced maturation of dendritic cells through the release of tumor antigens. This strategy to inhibit heat resistance in HIPEC presented an unprecedented paradigm for converting "cold" tumors into "hot" ones, thus significantly eradicating disseminated tumors located deep in the abdominal cavity and stimulating immune response in peritoneal metastases of a mouse model. Collectively, the nanoinhibitor effectively induced pyroptosis of colon tumor cells under heat conditions by inhibiting heat stress resistance and increasing oxidative stress, which may provide a new strategy for treatment of colorectal peritoneal metastases.
Keywords: Epigallocatechin gallate; Heat shock protein 90 inhibitor; Heat stress resistance; Hyperthermic intraperitoneal chemotherapy; Pyroptosis.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Upregulated Heat Shock Proteins After Hyperthermic Chemotherapy Point to Induced Cell Survival Mechanisms in Affected Tumor Cells From Peritoneal Carcinomatosis.Cancer Growth Metastasis. 2017 Sep 18;10:1179064417730559. doi: 10.1177/1179064417730559. eCollection 2017. Cancer Growth Metastasis. 2017. PMID: 29403306 Free PMC article.
-
[Construction and evaluation of a nomogram for predicting the prognosis of patients with colorectal cancer with peritoneal carcinomatosis treated with cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy].Zhonghua Wei Chang Wai Ke Za Zhi. 2023 May 25;26(5):434-441. doi: 10.3760/cma.j.cn441530-20230309-00071. Zhonghua Wei Chang Wai Ke Za Zhi. 2023. PMID: 37217351 Chinese.
-
Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): a multicentre, randomised, open-label, phase 3 trial.Lancet Oncol. 2021 Feb;22(2):256-266. doi: 10.1016/S1470-2045(20)30599-4. Epub 2021 Jan 18. Lancet Oncol. 2021. PMID: 33476595 Clinical Trial.
-
Hyperthermic Intraperitoneal Chemotherapy for Ovarian and Colorectal Cancer: A Review.JAMA Oncol. 2021 Aug 1;7(8):1231-1238. doi: 10.1001/jamaoncol.2021.0580. JAMA Oncol. 2021. PMID: 33956063 Review.
-
Direct and indirect anticancer effects of hyperthermic intraperitoneal chemotherapy on peritoneal malignancies (Review).Oncol Rep. 2021 Apr;45(4):23. doi: 10.3892/or.2021.7974. Epub 2021 Mar 2. Oncol Rep. 2021. PMID: 33649857 Review.
Cited by
-
Advances in targeted therapy for tumor with nanocarriers: A review.Mater Today Bio. 2025 Feb 15;31:101583. doi: 10.1016/j.mtbio.2025.101583. eCollection 2025 Apr. Mater Today Bio. 2025. PMID: 40061211 Free PMC article. Review.
-
Extracellular vesicle-mediated delivery of circp53 suppresses the progression of multiple cancers by activating the CypD/TRAP/HSP90 pathway.Exp Mol Med. 2025 Aug 1. doi: 10.1038/s12276-025-01506-0. Online ahead of print. Exp Mol Med. 2025. PMID: 40744997
-
Oxidative Stress and Redox Signaling in Gastric Cancer: From Mechanisms to Therapeutic Implications.Antioxidants (Basel). 2025 Feb 24;14(3):258. doi: 10.3390/antiox14030258. Antioxidants (Basel). 2025. PMID: 40227215 Free PMC article. Review.
-
Functional Materials Targeted Regulation of Gasdermins: From Fundamentals to Functionalities and Applications.Adv Sci (Weinh). 2025 Apr;12(16):e2500873. doi: 10.1002/advs.202500873. Epub 2025 Mar 24. Adv Sci (Weinh). 2025. PMID: 40273335 Free PMC article. Review.
-
OLFM4 promotes the progression of intestinal metaplasia through activation of the MYH9/GSK3β/β-catenin pathway.Mol Cancer. 2024 Jun 7;23(1):124. doi: 10.1186/s12943-024-02016-9. Mol Cancer. 2024. PMID: 38849840 Free PMC article.
References
-
- Gamboa AC, Zaidi MY, Lee RM, Speegle S, Switchenko JM, Lipscomb J, Cloyd JM, Ahmed A, Grotz T, Leiting J, Fournier K, Lee AJ, Dineen S, Powers BD, Lowy AM, Kotha NV, Clarke C, Gamblin TC, Patel SH, Lee TC, Lambert L, Hendrix RJ, Abbott DE, Vande Walle K, Lafaro K, Lee B, Johnston FM, Greer J, Russell MC, Staley CA, Maithel SK. Optimal Surveillance Frequency After CRS/HIPEC for Appendiceal and Colorectal Neoplasms: A Multi-institutional Analysis of the US HIPEC Collaborative. Ann Surg Oncol. 2020;27(1):134–146. - PMC - PubMed
-
- Klaver CEL, Wisselink DD, Punt CJA, Snaebjornsson P, Crezee J, Aalbers AGJ, Brandt A, Bremers AJA, Burger JWA, Fabry HFJ, Ferenschild F, Festen S, van Grevenstein WMU, Hemmer PHJ, de Hingh IHJT, Kok NFM, Musters GD, Schoonderwoerd L, Tuynman JB, van de Ven AWH, van Westreenen HL, Wiezer MJ, Zimmerman DDE, van Zweeden AA, Dijkgraaf MGW, Tanis PJ, COLOPEC Collaborators Group Adjuvant hyperthermic intraperitoneal chemotherapy in patients with locally advanced colon cancer (COLOPEC): a multicentre, open-label, randomised trial. Lancet Gastroenterol Hepatol. 2019;4(10):761–770. - PubMed
-
- Razenberg LG, van Gestel YR, Lemmens VE, de Hingh IH, Creemers GJ. Bevacizumab in addition to palliative chemotherapy for patients with peritoneal carcinomatosis of colorectal origin: a nationwide population-based study. Clin Colorectal Cancer. 2016;15(2):e41–46. - PubMed
-
- Morris VK, Kennedy EB, Baxter NN, Benson AB, 3rd, Cercek A, Cho M, Ciombor KK, Cremolini C, Davis A, Deming DA, Fakih MG, Gholami S, Hong TS, Jaiyesimi I, Klute K, Lieu C, Sanoff H, Strickler JH, White S, Willis JA, Eng C. Treatment of metastatic colorectal cancer: ASCO Guideline. J Clin Oncol. 2023;41(3):678–700. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

