Efficacy of COVID-19 mRNA vaccination in patients with autoimmune disorders: humoral and cellular immune response
- PMID: 37316832
- PMCID: PMC10266318
- DOI: 10.1186/s12916-023-02868-w
Efficacy of COVID-19 mRNA vaccination in patients with autoimmune disorders: humoral and cellular immune response
Abstract
Background: The impact of immunosuppressive therapies on the efficacy of vaccines to SARS-CoV-2 is not completely clarified. We analyzed humoral and T cell-mediated response after COVID-19 mRNA vaccine in immunosuppressed patients and patients with common variable immunodeficiency disease (CVID).
Patients: We enrolled 38 patients and 11 healthy sex- and age-matched controls (HC). Four patients were affected by CVID and 34 by chronic rheumatic diseases (RDs). All patients with RDs were treated by corticosteroid therapy and/or immunosuppressive treatment and/or biological drugs: 14 patients were treated with abatacept, 10 with rituximab, and 10 with tocilizumab.
Methods: Total antibody titer to SARS-CoV-2 spike protein was assessed by electrochemiluminescence immunoassay, CD4 and CD4-CD8 T cell-mediated immune response was analyzed by interferon-γ (IFN-γ) release assay, the production of IFN-γ-inducible (CXCL9 and CXCL10) and innate-immunity chemokines (MCP-1, CXCL8, and CCL5) by cytometric bead array after stimulation with different spike peptides. The expression of CD40L, CD137, IL-2, IFN-γ, and IL-17 on CD4 and CD8 T cells, evaluating their activation status, after SARS-CoV-2 spike peptides stimulation, was analyzed by intracellular flow cytometry staining. Cluster analysis identified cluster 1, namely the "high immunosuppression" cluster, and cluster 2, namely the "low immunosuppression" cluster.
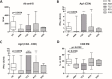
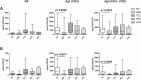
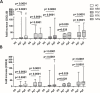
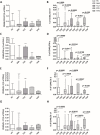
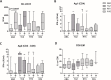
Results: After the second dose of vaccine, only abatacept-treated patients, compared to HC, showed a reduced anti-spike antibody response (mean: 432 IU/ml ± 562 vs mean: 1479 IU/ml ± 1051: p = 0.0034), and an impaired T cell response, compared with HC. In particular, we found a significantly reduced release of IFN-γ from CD4 and CD4-CD8 stimulated T cells, compared with HC (p = 0.0016 and p = 0.0078, respectively), reduced production of CXCL10 and CXCL9 from stimulated CD4 (p = 0.0048 and p = 0.001) and CD4-CD8 T cells (p = 0.0079 and p = 0.0006). Multivariable General Linear Model analysis confirmed a relationship between abatacept exposure and impaired production of CXCL9, CXCL10, and IFN-γ from stimulated T cells. Cluster analysis confirms that cluster 1 (including abatacept and half of rituximab treated cases) showed a reduced IFN-γ response, as well as reduced monocyte-derived chemokines All groups of patients demonstrated the ability to generate specific CD4 T activated cells after spike proteins stimulation. After the third dose of vaccine, abatacept-treated patients acquired the ability to produce a strong antibody response, showing an anti-S titer significantly higher compared to that obtained after the second dose (p = 0.0047), and comparable with the anti-S titer of the other groups.
Conclusions: Patients treated with abatacept showed an impaired humoral immune response to two doses of COVID-19 vaccine. The third vaccine dose has been demonstrated to be useful to induce a more robust antibody response to balance an impaired T cell-mediated one. All patients, exposed to different immunosuppressive drugs, were able to produce specific CD4-activated T cells, after spike proteins stimulation.
Trial registration: Local Ethical Committee NP4187.
Keywords: Abatacept; Autoimmune diseases; COVID-19 vaccination; Interferon-γ; Rituximab; T cell.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Kinetics of the B- and T-Cell Immune Responses After 6 Months From SARS-CoV-2 mRNA Vaccination in Patients With Rheumatoid Arthritis.Front Immunol. 2022 Feb 28;13:846753. doi: 10.3389/fimmu.2022.846753. eCollection 2022. Front Immunol. 2022. PMID: 35309297 Free PMC article.
-
Rituximab Impairs B Cell Response But Not T Cell Response to COVID-19 Vaccine in Autoimmune Diseases.Arthritis Rheumatol. 2022 Jun;74(6):927-933. doi: 10.1002/art.42058. Epub 2022 Mar 29. Arthritis Rheumatol. 2022. PMID: 34962357 Free PMC article.
-
Humoral- and T-Cell-Specific Immune Responses to SARS-CoV-2 mRNA Vaccination in Patients With MS Using Different Disease-Modifying Therapies.Neurology. 2022 Feb 1;98(5):e541-e554. doi: 10.1212/WNL.0000000000013108. Epub 2021 Nov 22. Neurology. 2022. PMID: 34810244 Free PMC article.
-
Easy approach to detect cell immunity to COVID vaccines in common variable immunodeficiency patients.Allergol Immunopathol (Madr). 2022 May 1;50(3):101-105. doi: 10.15586/aei.v50i3.583. eCollection 2022. Allergol Immunopathol (Madr). 2022. PMID: 35527662 Review.
-
Immunosuppressed non-responders to two doses of mRNA SARS-CoV-2 vaccines achieve an immune response comparable to those of immunocompetent individuals after a third dose.Hormones (Athens). 2022 Sep;21(3):369-373. doi: 10.1007/s42000-022-00365-y. Epub 2022 Jun 24. Hormones (Athens). 2022. PMID: 35750960 Free PMC article. Review.
Cited by
-
Safety, efficacy, and immunogenicity of SARS-CoV-2 mRNA vaccination in children and adult patients with rheumatic diseases: a comprehensive literature review.Rheumatol Int. 2024 Dec;44(12):2757-2794. doi: 10.1007/s00296-024-05734-x. Epub 2024 Nov 22. Rheumatol Int. 2024. PMID: 39576327
-
Connective tissue disorders in COVID-19: Reply to "People with a connective tissue disorder may be especially vulnerable to the endothelial damage that characterizes long COVID due to the fragility of their vasculature and slow wound healing".Angiogenesis. 2024 May;27(2):125-127. doi: 10.1007/s10456-024-09915-x. Epub 2024 Mar 26. Angiogenesis. 2024. PMID: 38532037
-
Cellular immune response to the anti-SARS-CoV-2 BNT162b2 mRNA vaccine in pediatric autoimmune inflammatory rheumatic disease patients and controls.Clin Exp Immunol. 2024 Jul 12;217(2):167-172. doi: 10.1093/cei/uxae044. Clin Exp Immunol. 2024. PMID: 38767466 Free PMC article.
-
COVID-19 Vaccination and Immunosuppressive Therapy in Immune-Mediated Inflammatory Diseases.Vaccines (Basel). 2023 Dec 4;11(12):1813. doi: 10.3390/vaccines11121813. Vaccines (Basel). 2023. PMID: 38140217 Free PMC article. Review.
-
Effectiveness of a fourth dose of COVID-19 mRNA vaccine in patients with systemic autoimmune rheumatic diseases using disease-modifying antirheumatic drugs: an emulated target trial.Lancet Rheumatol. 2024 Jan;6(1):e21-e30. doi: 10.1016/S2665-9913(23)00272-2. Epub 2023 Nov 15. Lancet Rheumatol. 2024. PMID: 38258675 Free PMC article. Clinical Trial.
References
-
- Strangfeld A, Schäfer M, Gianfrancesco MA, Lawson-Tovey S, Liew JW, Ljung L, et al. COVID-19 Global Rheumatology Alliance. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2021;80:930–942. doi: 10.1136/annrheumdis-2020-219498. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

