Lactobacillus acidophilus and propionate attenuate Sjögren's syndrome by modulating the STIM1-STING signaling pathway
- PMID: 37316856
- PMCID: PMC10265917
- DOI: 10.1186/s12964-023-01141-0
Lactobacillus acidophilus and propionate attenuate Sjögren's syndrome by modulating the STIM1-STING signaling pathway
Abstract
Background: Sjögren's syndrome (SS) is an autoimmune disease characterized by inflammation of the exocrine gland. An imbalance of gut microbiota has been linked to SS. However, the molecular mechanism is unclear. We investigated the effects of Lactobacillus acidophilus (L. acidophilus) and propionate on the development and progression of SS in mouse model.
Methods: We compared the gut microbiomes of young and old mice. We administered L. acidophilus and propionate up to 24 weeks. The saliva flow rate and the histopathology of the salivary glands were investigated, and the effects of propionate on the STIM1-STING signaling pathway were evaluated in vitro.
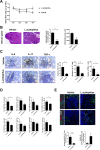
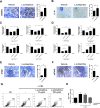
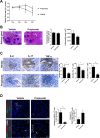
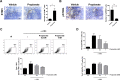
Results: Lactobacillaceae and Lactobacillus were decreased in aged mice. SS symptoms were ameliorated by L. acidophilus. The abundance of propionate-producing bacterial was increased by L. acidophilus. Propionate ameliorated the development and progression of SS by inhibiting the STIM1-STING signaling pathway.
Conclusions: The findings suggest that Lactobacillus acidophilus and propionate have therapeutic potential for SS. Video Abstract.
Keywords: Lactobacillus acidophilus; Propionate; Sjögren’s syndrome; Stimulation of interferon genes (STING); Stromal interaction moleculae 1 (STIM1).
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

