Adsorption of Cd (II) by a novel living and non-living Cupriavidus necator GX_5: optimization, equilibrium and kinetic studies
- PMID: 37316907
- PMCID: PMC10265800
- DOI: 10.1186/s13065-023-00977-4
Adsorption of Cd (II) by a novel living and non-living Cupriavidus necator GX_5: optimization, equilibrium and kinetic studies
Abstract
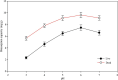
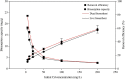
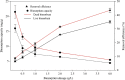
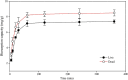
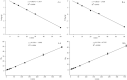
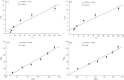
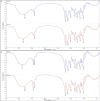
Biosorbents have been extensively studied for heavy metal adsorption due to their advantages of low cost and high efficiency. In the study, the living and non-living biomass of Cupriavidus necator GX_5 previously isolated were evaluated for their adsorption capacity and/or removal efficiency for Cd (II) through batch experiments, SEM and FT-IR investigations. The maximum removal efficiency rates for the live and dead biomass were 60.51% and 78.53%, respectively, at an optimum pH of 6, a dosage of 1 g/L and an initial Cd (II) concentration of 5 mg/L. The pseudo-second-order kinetic model was more suitable for fitting the experimental data, indicating that the rate-limiting step might be chemisorption. The Freundlich isotherm model fit better than the Langmuir isotherm model, implying that the adsorption process of both biosorbents was heterogeneous. FT-IR observation reflected that various functional groups were involved in Cd (II) adsorption: -OH, -NH, C=O, C-O and C-C groups for the living biomass and -OH, -NH, C-H, C = O, C-N and N-H groups for the dead biomass. Our results imply that non-living biosorbents have a higher capacity and stronger strength for absorbing Cd (II) than living biomass. Therefore, we suggest that dead GX_5 is a promising adsorbent and can be used in Cd (II)-contaminated environments.
Keywords: Adsorption; Biosorbent; Cadmium; Cupriavidus necator GX_5.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Adsorption of cadmium by live and dead biomass of plant growth-promoting rhizobacteria.RSC Adv. 2018 Oct 1;8(58):33523-33533. doi: 10.1039/c8ra06758a. eCollection 2018 Sep 24. RSC Adv. 2018. PMID: 35548138 Free PMC article.
-
Carnauba (Copernicia prunifera) palm tree biomass as adsorbent for Pb(II) and Cd(II) from water medium.Environ Sci Pollut Res Int. 2021 Apr;28(15):18941-18952. doi: 10.1007/s11356-020-07635-5. Epub 2020 Jan 14. Environ Sci Pollut Res Int. 2021. PMID: 31933097
-
Biosorption and bioaccumulation characteristics of cadmium by plant growth-promoting rhizobacteria.RSC Adv. 2018 Sep 3;8(54):30902-30911. doi: 10.1039/c8ra06270f. eCollection 2018 Aug 30. RSC Adv. 2018. PMID: 35548749 Free PMC article.
-
Cadmium resistance, microbial biosorptive performance and mechanisms of a novel biocontrol bacterium Paenibacillus sp. LYX-1.Environ Sci Pollut Res Int. 2022 Sep;29(45):68692-68706. doi: 10.1007/s11356-022-20581-8. Epub 2022 May 11. Environ Sci Pollut Res Int. 2022. PMID: 35543785
-
Characterization of Cd2+ biosorption by Pseudomonas sp. strain 375, a novel biosorbent isolated from soil polluted with heavy metals in Southern China.Chemosphere. 2020 Feb;240:124893. doi: 10.1016/j.chemosphere.2019.124893. Epub 2019 Sep 17. Chemosphere. 2020. PMID: 31550585
Cited by
-
The molecular insights of cyanobacterial bioremediations of heavy metals: the current and the future challenges.Front Microbiol. 2024 Oct 11;15:1450992. doi: 10.3389/fmicb.2024.1450992. eCollection 2024. Front Microbiol. 2024. PMID: 39464393 Free PMC article. Review.
-
Phylotaxonomic analysis uncovers underestimated species diversity within the genus Rossellomorea with description of selenium-resistant Rossellomorea yichunensis sp. nov.BMC Microbiol. 2025 Jul 3;25(1):408. doi: 10.1186/s12866-025-04138-6. BMC Microbiol. 2025. PMID: 40610858 Free PMC article.
-
Response surface optimization for cadmium biosorption onto the pre-treated biomass of red algae Digenia simplex as a sustainable indigenous biosorbent.PeerJ. 2025 Aug 4;13:e19776. doi: 10.7717/peerj.19776. eCollection 2025. PeerJ. 2025. PMID: 40777079 Free PMC article.
References
-
- Ali H, Khan E. What are heavy metals? long-standing controversy over the scientific use of the term ‘heavy metals’–proposal of a comprehensive definition. Toxicol Environ Chem. 2018;100:6–19. doi: 10.1080/02772248.2017.1413652. - DOI
-
- Cui L, Li J, Gao X, Tian B, Zhang J, Wang X, Liu Z. Human health ambient water quality criteria for 13 heavy metals and health risk assessment in Taihu Lake. Front Environ Sci Eng. 2022;16(4):41. doi: 10.1007/s11783-021-1475-6. - DOI
-
- Plöhn M, Escudero-Oñate C, Funk C. Biosorption of Cd (II) by nordic microalgae: tolerance, kinetics and equilibrium studies. Algal Res. 2021;59:102471. doi: 10.1016/j.algal.2021.102471. - DOI
Grants and funding
- 32260033/National Natural Science Foundation of China
- GJJ201619/Science and Technology Research Project of Jiangxi Education Department
- GJJ201618/Science and Technology Research Project of Jiangxi Education Department
- S202110417021/Innovation and Entrepreneurship Training Program for College Students in Jiangxi Province
LinkOut - more resources
Full Text Sources
