Bioactivity of human adult stem cells and functional relevance of stem cell-derived extracellular matrix in chondrogenesis
- PMID: 37316923
- PMCID: PMC10268391
- DOI: 10.1186/s13287-023-03392-7
Bioactivity of human adult stem cells and functional relevance of stem cell-derived extracellular matrix in chondrogenesis
Abstract
Background: Autologous chondrocyte implantation (ACI) has been used to treat articular cartilage defects for over two decades. Adult stem cells have been proposed as a solution to inadequate donor cell numbers often encountered in ACI. Multipotent stem/progenitor cells isolated from adipose, bone marrow, and cartilage are the most promising cell therapy candidates. However, different essential growth factors are required to induce these tissue-specific stem cells to initiate chondrogenic differentiation and subsequent deposition of extracellular matrix (ECM) to form cartilage-like tissue. Upon transplantation into cartilage defects in vivo, the levels of growth factors in the host tissue are likely to be inadequate to support chondrogenesis of these cells in situ. The contribution of stem/progenitor cells to cartilage repair and the quality of ECM produced by the implanted cells required for cartilage repair remain largely unknown. Here, we evaluated the bioactivity and chondrogenic induction ability of the ECM produced by different adult stem cells.
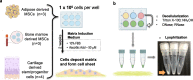
Methods: Adult stem/progenitor cells were isolated from human adipose (hADSCs), bone marrow (hBMSCs), and articular cartilage (hCDPCs) and cultured for 14 days in monolayer in mesenchymal stromal cell (MSC)-ECM induction medium to allow matrix deposition and cell sheet formation. The cell sheets were then decellularized, and the protein composition of the decellularized ECM (dECM) was analyzed by BCA assay, SDS-PAGE, and immunoblotting for fibronectin (FN), collagen types I (COL1) and III (COL3). The chondrogenic induction ability of the dECM was examined by seeding undifferentiated hBMSCs onto the respective freeze-dried solid dECM followed by culturing in serum-free medium for 7 days. The expression levels of chondrogenic genes SOX9, COL2, AGN, and CD44 were analyzed by q-PCR.
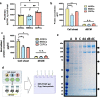
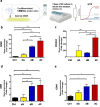
Results: hADSCs, hBMSCs, and hCDPCs generated different ECM protein profiles and exhibited significantly different chondrogenic effects. hADSCs produced 20-60% more proteins than hBMSCs and hCDPCs and showed a fibrillar-like ECM pattern (FNhigh, COL1high). hCDPCs produced more COL3 and deposited less FN and COL1 than the other cell types. The dECM derived from hBMSCs and hCDPCs induced spontaneous chondrogenic gene expression in hBMSCs.
Conclusions: These findings provide new insights on application of adult stem cells and stem cell-derived ECM to enhance cartilage regeneration.
Keywords: Adipose-derived stem cells; Adult stem cells; Bone marrow-derived stem cells; Cartilage repair; Cartilage-derived stem/progenitor cells; Chondrogenesis; Extracellular matrix.
© 2023. The Author(s).
Conflict of interest statement
YJ, none. RST serves as one of the Editors-in-Chiefs of
Figures


Similar articles
-
Mesenchymal stem cell-derived extracellular matrix enhances chondrogenic phenotype of and cartilage formation by encapsulated chondrocytes in vitro and in vivo.Acta Biomater. 2018 Mar 15;69:71-82. doi: 10.1016/j.actbio.2017.12.043. Epub 2018 Jan 6. Acta Biomater. 2018. PMID: 29317369 Free PMC article.
-
Prochondrogenic effect of decellularized extracellular matrix secreted from human induced pluripotent stem cell-derived chondrocytes.Acta Biomater. 2023 Sep 1;167:234-248. doi: 10.1016/j.actbio.2023.05.052. Epub 2023 Jun 8. Acta Biomater. 2023. PMID: 37295627
-
Cartilaginous extracellular matrix derived from decellularized chondrocyte sheets for the reconstruction of osteochondral defects in rabbits.Acta Biomater. 2018 Nov;81:129-145. doi: 10.1016/j.actbio.2018.10.005. Epub 2018 Oct 6. Acta Biomater. 2018. PMID: 30300711
-
Cell-derived decellularized extracellular matrix scaffolds for articular cartilage repair.Int J Artif Organs. 2021 Apr;44(4):269-281. doi: 10.1177/0391398820953866. Epub 2020 Sep 18. Int J Artif Organs. 2021. PMID: 32945220 Review.
-
The Challenge in Using Mesenchymal Stromal Cells for Recellularization of Decellularized Cartilage.Stem Cell Rev Rep. 2017 Feb;13(1):50-67. doi: 10.1007/s12015-016-9699-8. Stem Cell Rev Rep. 2017. PMID: 27826794 Review.
Cited by
-
Stem cell therapy for intervertebral disc degeneration: Clinical progress with exosomes and gene vectors.World J Stem Cells. 2025 Apr 26;17(4):102945. doi: 10.4252/wjsc.v17.i4.102945. World J Stem Cells. 2025. PMID: 40308883 Free PMC article. Review.
-
The considerations on selecting the appropriate decellularized ECM for specific regeneration demands.Mater Today Bio. 2024 Oct 18;29:101301. doi: 10.1016/j.mtbio.2024.101301. eCollection 2024 Dec. Mater Today Bio. 2024. PMID: 39498148 Free PMC article. Review.
-
Senescence-Associated Alterations in Matrisome of Mesenchymal Stem Cells.Int J Mol Sci. 2024 May 14;25(10):5332. doi: 10.3390/ijms25105332. Int J Mol Sci. 2024. PMID: 38791371 Free PMC article.
-
Cartilage stem/progenitor cells-derived exosomes facilitate knee cartilage repair in a subacute osteoarthritis rat model.J Cell Mol Med. 2024 Apr;28(8):e18327. doi: 10.1111/jcmm.18327. J Cell Mol Med. 2024. PMID: 38661437 Free PMC article.
-
Injectable and Self-Curing Single-Component Hydrogel for Stem Cell Encapsulation and In Vivo Bone Regeneration.Adv Sci (Weinh). 2024 Apr;11(16):e2304861. doi: 10.1002/advs.202304861. Epub 2024 Feb 14. Adv Sci (Weinh). 2024. PMID: 38355304 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

