Cartilage regeneration and inflammation modulation in knee osteoarthritis following injection of allogeneic adipose-derived mesenchymal stromal cells: a phase II, triple-blinded, placebo controlled, randomized trial
- PMID: 37316949
- PMCID: PMC10268462
- DOI: 10.1186/s13287-023-03359-8
Cartilage regeneration and inflammation modulation in knee osteoarthritis following injection of allogeneic adipose-derived mesenchymal stromal cells: a phase II, triple-blinded, placebo controlled, randomized trial
Abstract
Background: Intra-articular injection of mesenchymal stromal cells (MSCs) with immunomodulatory features and their paracrine secretion of regenerative factors proposed a noninvasive therapeutic modality for cartilage regeneration in knee osteoarthritis (KOA).
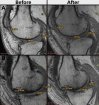
Methods: Total number of 40 patients with KOA enrolled in two groups. Twenty patients received intra-articular injection of 100 × 106 allogeneic adipose-derived mesenchymal stromal cells (AD-MSCs), and 20 patients as control group received placebo (normal saline). Questionnaire-based measurements, certain serum biomarkers, and some cell surface markers were evaluated for 1 year. Magnetic resonance imaging (MRI) before and 1 year after injection was performed to measure possible changes in the articular cartilage.
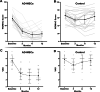
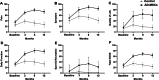
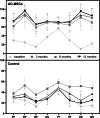
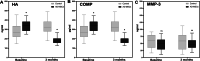
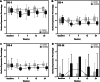
Results: Forty patients allocated including 4 men (10%) and 36 women (90%) with average age of 56.1 ± 7.2 years in control group and 52.8 ± 7.5 years in AD-MSCs group. Four patients (two patients from AD-MSCs group and two patients from the control group) excluded during the study. Clinical outcome measures showed improvement in AD-MSCs group. Hyaluronic acid and cartilage oligomeric matrix protein levels in blood serum decreased significantly in patients who received AD-MSCs (P < 0.05). Although IL-10 level significantly increased after 1 week (P < 0.05), the serum level of inflammatory markers dramatically decreased after 3 months (P < 0.001). Expressions of CD3, CD4, and CD8 have a decreasing trend during 6-month follow-up (P < 0.05), (P < 0.001), and (P < 0.001), respectively. However, the number of CD25+ cells increased remarkably in the treatment group 3 months after intervention (P < 0.005). MRI findings showed a slight increase in the thickness of tibial and femoral articular cartilages in AD-MSCs group. The changes were significant in the medial posterior and medial anterior areas of the tibia with P < 0.01 and P < 0.05, respectively.
Conclusion: Inter-articular injection of AD-MSCs in patients with KOA is safe. Laboratory data, MRI findings, and clinical examination of patients at different time points showed notable articular cartilage regeneration and significant improvement in the treatment group.
Trial registration: Iranian registry of clinical trials (IRCT, https://en.irct.ir/trial/46 ), IRCT20080728001031N23. Registered 24 April 2018.
Keywords: Cartilage regeneration; Cell-based therapy; Knee osteoarthritis; Liquid biomarkers; Mesenchymal stromal cells; Regenerative medicine.
© 2023. The Author(s).
Conflict of interest statement
MV is the regulatory affairs manager at the cell production facility. He has no share in the company nor any financial benefit in this study. The other authors declare that they have no competing interests.
Figures




Similar articles
-
Clinical and laboratory findings following transplantation of allogeneic adipose-derived mesenchymal stromal cells in knee osteoarthritis, a brief report.Connect Tissue Res. 2022 Nov;63(6):663-674. doi: 10.1080/03008207.2022.2074841. Epub 2022 Jul 20. Connect Tissue Res. 2022. PMID: 35856397 Clinical Trial.
-
Intra-Articular Injection of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis: A Phase IIb, Randomized, Placebo-Controlled Clinical Trial.Stem Cells Transl Med. 2019 Jun;8(6):504-511. doi: 10.1002/sctm.18-0122. Epub 2019 Mar 5. Stem Cells Transl Med. 2019. PMID: 30835956 Free PMC article. Clinical Trial.
-
Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial.Stem Cells. 2014 May;32(5):1254-66. doi: 10.1002/stem.1634. Stem Cells. 2014. PMID: 24449146 Clinical Trial.
-
Intraarticular injection of bone marrow-derived mesenchymal stem cells enhances regeneration in knee osteoarthritis.Knee Surg Sports Traumatol Arthrosc. 2020 Dec;28(12):3827-3842. doi: 10.1007/s00167-020-05859-z. Epub 2020 Jan 31. Knee Surg Sports Traumatol Arthrosc. 2020. PMID: 32006075 Free PMC article. Review.
-
Intra-articular Injection of Autologous Adipose-Derived Stem Cells or Stromal Vascular Fractions: Are They Effective for Patients With Knee Osteoarthritis? A Systematic Review With Meta-analysis of Randomized Controlled Trials.Am J Sports Med. 2023 Mar;51(3):837-848. doi: 10.1177/03635465211053893. Epub 2022 Jan 12. Am J Sports Med. 2023. PMID: 35019764
Cited by
-
A Phase I Dose-Escalation Clinical Trial to Assess the Safety and Efficacy of Umbilical Cord-Derived Mesenchymal Stromal Cells in Knee Osteoarthritis.Stem Cells Transl Med. 2024 Mar 15;13(3):193-203. doi: 10.1093/stcltm/szad088. Stem Cells Transl Med. 2024. PMID: 38366909 Free PMC article. Clinical Trial.
-
USP15-modified ADMSCs-Exo alleviates chondrocyte damage and effectively relieved osteoarthritis by inducing M2 polarization of macrophages through deubiquitinating FOXC1.J Orthop Surg Res. 2025 Apr 2;20(1):336. doi: 10.1186/s13018-025-05742-y. J Orthop Surg Res. 2025. PMID: 40176111 Free PMC article.
-
Targeted therapy for knee osteoarthritis: From basic to clinics.Medicine (Baltimore). 2025 Aug 15;104(33):e43686. doi: 10.1097/MD.0000000000043686. Medicine (Baltimore). 2025. PMID: 40826764 Free PMC article. Review.
-
Impact of adipose-derived mesenchymal stem cells and their secretome on osteoarthritis in a rat model.BMC Musculoskelet Disord. 2025 Apr 21;26(1):392. doi: 10.1186/s12891-025-08642-8. BMC Musculoskelet Disord. 2025. PMID: 40259333 Free PMC article.
-
Osteoarthritis: Mechanisms and Therapeutic Advances.MedComm (2020). 2025 Aug 1;6(8):e70290. doi: 10.1002/mco2.70290. eCollection 2025 Aug. MedComm (2020). 2025. PMID: 40757100 Free PMC article. Review.
References
-
- Wilson R, Abbott JH. The projected burden of knee osteoarthritis in New Zealand: healthcare expenditure and total joint replacement provision. N Z Med J. 2019;132(1503):53–65. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

