Oral Administration of Lacticaseibacillus rhamnosus CRL1505 Modulates Lung Innate Immune Response against Klebsiella pneumoniae ST25
- PMID: 37317122
- PMCID: PMC10222716
- DOI: 10.3390/microorganisms11051148
Oral Administration of Lacticaseibacillus rhamnosus CRL1505 Modulates Lung Innate Immune Response against Klebsiella pneumoniae ST25
Abstract
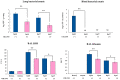
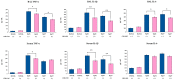
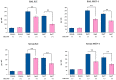
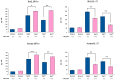
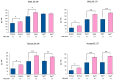
Orally administered Lacticaseibacillus rhamnosus CRL1505 enhances respiratory immunity, providing protection against respiratory viruses and Streptococcus pneumoniae. However, the capacity of the CRL1505 strain to improve respiratory immunity against Gram-negative bacterial infections has not been evaluated before. The aim of this work was to evaluate whether the Lcb. rhamnosus CRL1505 was able to beneficially regulate the respiratory innate immune response and enhance the resistance to hypermucoviscous KPC-2-producing Klebsiella pneumoniae of the sequence type 25 (ST25). BALB/c mice were treated with the CRL1505 strain via the oral route and then nasally challenged with K. pneumoniae ST25 strains LABACER 01 or LABACER 27. Bacterial cell counts, lung injuries and the respiratory and systemic innate immune responses were evaluated after the bacterial infection. The results showed that K. pneumoniae ST25 strains increased the levels of TNF-α, IL-1β, IL-6, IFN-γ, IL-17, KC and MPC-1 in the respiratory tract and blood, as well as the numbers of BAL neutrophils and macrophages. Mice treated with Lcb. rhamnosus CRL1505 had significantly lower K. pneumoniae counts in their lungs, as well as reduced levels of inflammatory cells, cytokines and chemokines in the respiratory tract and blood when compared to infected controls. Furthermore, higher levels of the regulatory cytokines IL-10 and IL-27 were found in the respiratory tract and blood of CRL1505-treated mice than controls. These results suggest that the ability of Lcb. rhamnosus CRL1505 to help with the control of detrimental inflammation in lungs during K. pneumoniae infection would be a key feature to improve the resistance to this pathogen. Although further mechanistic studies are necessary, Lcb. rhamnosus CRL1505 can be proposed as a candidate to improve patients' protection against hypermucoviscous KPC-2-producing strains belonging to the ST25, which is endemic in the hospitals of our region.
Keywords: K. pneumoniae; Lcb. rhamnosus CRL1505; innate immunity; probiotics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Gomez S.A., Pasteran F.G., Faccone D., Tijet N., Rapoport M., Lucero C., Lastovetska O., Albornoz E., Galas M., Melano R.G., et al. Clonal dissemination of Klebsiella pneumoniae ST258 harbouring KPC-2 in Argentina. Clin. Microbiol. Infect. 2011;17:1520–1524. doi: 10.1111/j.1469-0691.2011.03600.x. - DOI - PubMed
-
- Cejas D., Fernandez Canigia L., Nastro M., Rodríguez C., Tanco A., Rodríguez H., Vay C., Maldonado I., Famiglietti A., Giovanakis M., et al. Hyperendemic clone of KPC producing Klebsiella pneumoniae ST 258 in Buenos Aires hospitals. Infect. Genet. Evol. 2012;12:499–501. doi: 10.1016/j.meegid.2011.09.018. - DOI - PubMed
-
- Cejas D., Elena A., Guevara Nuñez D., Sevilla Platero P., De Paulis A., Magariños F., Alfonso C., Berger M.A., Fernández-Canigia L., Gutkind G., et al. Changing epidemiology of KPC-producing Klebsiella pneumoniae in Argentina: Emergence of hypermucoviscous ST25 and high-risk clone ST307. J. Glob. Antimicrob. Resist. 2019;18:238–242. doi: 10.1016/j.jgar.2019.06.005. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

