Suppressive Effects of Volatile Compounds from Bacillus spp. on Magnaporthe oryzae Triticum (MoT) Pathotype, Causal Agent of Wheat Blast
- PMID: 37317265
- PMCID: PMC10221767
- DOI: 10.3390/microorganisms11051291
Suppressive Effects of Volatile Compounds from Bacillus spp. on Magnaporthe oryzae Triticum (MoT) Pathotype, Causal Agent of Wheat Blast
Abstract
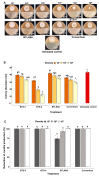
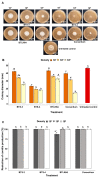
The Magnaporthe oryzae Triticum (MoT) pathotype is the causal agent of wheat blast, which has caused significant economic losses and threatens wheat production in South America, Asia, and Africa. Three bacterial strains from rice and wheat seeds (B. subtilis BTS-3, B. velezensis BTS-4, and B. velezensis BTLK6A) were used to explore the antifungal effects of volatile organic compounds (VOCs) of Bacillus spp. as a potential biocontrol mechanism against MoT. All bacterial treatments significantly inhibited both the mycelial growth and sporulation of MoT in vitro. We found that this inhibition was caused by Bacillus VOCs in a dose-dependent manner. In addition, biocontrol assays using detached wheat leaves infected with MoT showed reduced leaf lesions and sporulation compared to the untreated control. VOCs from B. velezensis BTS-4 alone or a consortium (mixture of B. subtilis BTS-3, B. velezensis BTS-4, and B. velezensis BTLK6A) of treatments consistently suppressed MoT in vitro and in vivo. Compared to the untreated control, VOCs from BTS-4 and the Bacillus consortium reduced MoT lesions in vivo by 85% and 81.25%, respectively. A total of thirty-nine VOCs (from nine different VOC groups) from four Bacillus treatments were identified by gas chromatography-mass spectrometry (GC-MS), of which 11 were produced in all Bacillus treatments. Alcohols, fatty acids, ketones, aldehydes, and S-containing compounds were detected in all four bacterial treatments. In vitro assays using pure VOCs revealed that hexanoic acid, 2-methylbutanoic acid, and phenylethyl alcohol are potential VOCs emitted by Bacillus spp. that are suppressive for MoT. The minimum inhibitory concentrations for MoT sporulation were 250 mM for phenylethyl alcohol and 500 mM for 2-methylbutanoic acid and hexanoic acid. Therefore, our results indicate that VOCs from Bacillus spp. are effective compounds to suppress the growth and sporulation of MoT. Understanding the MoT sporulation reduction mechanisms exerted by Bacillus VOCs may provide novel options to manage the further spread of wheat blast by spores.
Keywords: Bacillus; GC–MS; biocontrol; sporulation; volatile organic compound.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Biological control potential of worrisome wheat blast disease by the seed endophytic bacilli.Front Microbiol. 2024 Mar 11;15:1336515. doi: 10.3389/fmicb.2024.1336515. eCollection 2024. Front Microbiol. 2024. PMID: 38529179 Free PMC article.
-
Inhibitory Effects of Linear Lipopeptides From a Marine Bacillus subtilis on the Wheat Blast Fungus Magnaporthe oryzae Triticum.Front Microbiol. 2020 Apr 30;11:665. doi: 10.3389/fmicb.2020.00665. eCollection 2020. Front Microbiol. 2020. PMID: 32425899 Free PMC article.
-
Oligomycins inhibit Magnaporthe oryzae Triticum and suppress wheat blast disease.PLoS One. 2020 Aug 17;15(8):e0233665. doi: 10.1371/journal.pone.0233665. eCollection 2020. PLoS One. 2020. PMID: 32804955 Free PMC article.
-
Wheat Blast: A Disease Spreading by Intercontinental Jumps and Its Management Strategies.Front Plant Sci. 2021 Jul 23;12:710707. doi: 10.3389/fpls.2021.710707. eCollection 2021. Front Plant Sci. 2021. PMID: 34367228 Free PMC article. Review.
-
Wheat spike blast: genetic interventions for effective management.Mol Biol Rep. 2022 Jun;49(6):5483-5494. doi: 10.1007/s11033-022-07356-7. Epub 2022 Apr 27. Mol Biol Rep. 2022. PMID: 35478296 Review.
Cited by
-
Screening and characterization of biocontrol bacteria isolated from Ageratum conyzoides against Collectotrichum fructicola causing Chinese plum (Prunus salicina Lindl.) anthracnose.Front Microbiol. 2023 Dec 7;14:1296755. doi: 10.3389/fmicb.2023.1296755. eCollection 2023. Front Microbiol. 2023. PMID: 38130944 Free PMC article.
-
Bacillus amyloliquefaciens LM-1 Affects Multiple Cell Biological Processes in Magnaporthe oryzae to Suppress Rice Blast.Microorganisms. 2024 Jun 20;12(6):1246. doi: 10.3390/microorganisms12061246. Microorganisms. 2024. PMID: 38930628 Free PMC article.
-
Microbiome Engineering for Sustainable Rice Production: Strategies for Biofertilization, Stress Tolerance, and Climate Resilience.Microorganisms. 2025 Jan 22;13(2):233. doi: 10.3390/microorganisms13020233. Microorganisms. 2025. PMID: 40005600 Free PMC article. Review.
-
"Stop, Little Pot" as the Motto of Suppressive Management of Various Microbial Consortia.Microorganisms. 2024 Aug 12;12(8):1650. doi: 10.3390/microorganisms12081650. Microorganisms. 2024. PMID: 39203492 Free PMC article. Review.
-
2-Nonanol produced by Bacillus velezensis EM-1: a new biocontrol agent against tobacco brown spot.Front Microbiol. 2025 Apr 30;16:1582372. doi: 10.3389/fmicb.2025.1582372. eCollection 2025. Front Microbiol. 2025. PMID: 40371117 Free PMC article.
References
-
- Awika J.M. Major cereal grains production and use around the world. In: Awika J.M., Piironen V., Bean S., editors. Advances in Cereal Science: Implications to Food Processing and Health Promotion. Volume 1089. American Chemical Society, ACS Symposium Series 1089; Washington, DC, USA: 2011. pp. 1–13. - DOI
-
- World Agricultural Production.com. [(accessed on 12 April 2023)]. Available online: www.worldagriculturalproduction.com/crops/wheat.aspx.
-
- Cruz C.D., Valent B. Wheat blast disease: Danger on the move. Trop. Plant Pathol. 2017;42:210–222. doi: 10.1007/s40858-017-0159-z. - DOI
-
- Ceresini P.C., Castroagudín V.L., Rodrigues F., Rios J.A., Aucique-Pérez C.E., Moreira S.I., Croll D., Alves E., de Carvalho G., Maciel J.L.N., et al. Wheat blast: From its origins in South America to its emergence as a global threat. Mol. Plant Pathol. 2018;20:155–172. doi: 10.1111/mpp.12747. - DOI - PMC - PubMed
-
- Islam T., Ansary M.W.R., Rahman M.M. Magnaporthe oryzae and its pathotypes: A potential plant pandemic threat to global food security. In: Scott B., Mesarich C., editors. Plant Relationships. Volume 5. Springer; Cham, Switzerland: 2023. pp. 425–462. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

