Interkingdom Detection of Bacterial Quorum-Sensing Molecules by Mammalian Taste Receptors
- PMID: 37317269
- PMCID: PMC10221136
- DOI: 10.3390/microorganisms11051295
Interkingdom Detection of Bacterial Quorum-Sensing Molecules by Mammalian Taste Receptors
Abstract
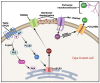
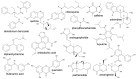
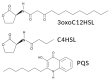
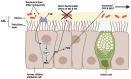
Bitter and sweet taste G protein-coupled receptors (known as T2Rs and T1Rs, respectively) were originally identified in type II taste cells on the tongue, where they signal perception of bitter and sweet tastes, respectively. Over the past ~15 years, taste receptors have been identified in cells all over the body, demonstrating a more general chemosensory role beyond taste. Bitter and sweet taste receptors regulate gut epithelial function, pancreatic β cell secretion, thyroid hormone secretion, adipocyte function, and many other processes. Emerging data from a variety of tissues suggest that taste receptors are also used by mammalian cells to "eavesdrop" on bacterial communications. These receptors are activated by several quorum-sensing molecules, including acyl-homoserine lactones and quinolones from Gram-negative bacteria such as Pseudomonas aeruginosa, competence stimulating peptides from Streptococcus mutans, and D-amino acids from Staphylococcus aureus. Taste receptors are an arm of immune surveillance similar to Toll-like receptors and other pattern recognition receptors. Because they are activated by quorum-sensing molecules, taste receptors report information about microbial population density based on the chemical composition of the extracellular environment. This review summarizes current knowledge of bacterial activation of taste receptors and identifies important questions remaining in this field.
Keywords: Pseudomonas aeruginosa; Staphylococcus aureus; Streptococcus mutans; acyl-homoserine lactone; cilia; competence-stimulating peptide; gingival epithelial cells; nasal epithelium; quinolone; solitary chemosensory cell.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in manuscript preparation, interpretation of prior studies, writing of the manuscript, or decision to publish.
Figures

Similar articles
-
Activation of airway epithelial bitter taste receptors by Pseudomonas aeruginosa quinolones modulates calcium, cyclic-AMP, and nitric oxide signaling.J Biol Chem. 2018 Jun 22;293(25):9824-9840. doi: 10.1074/jbc.RA117.001005. Epub 2018 May 10. J Biol Chem. 2018. PMID: 29748385 Free PMC article.
-
Bacterial d-amino acids suppress sinonasal innate immunity through sweet taste receptors in solitary chemosensory cells.Sci Signal. 2017 Sep 5;10(495):eaam7703. doi: 10.1126/scisignal.aam7703. Sci Signal. 2017. PMID: 28874606 Free PMC article.
-
Characterization of the Binding Sites for Bacterial Acyl Homoserine Lactones (AHLs) on Human Bitter Taste Receptors (T2Rs).ACS Infect Dis. 2018 Jul 13;4(7):1146-1156. doi: 10.1021/acsinfecdis.8b00094. Epub 2018 Jun 6. ACS Infect Dis. 2018. PMID: 29799189
-
The emerging role of the bitter taste receptor T2R38 in upper respiratory infection and chronic rhinosinusitis.Am J Rhinol Allergy. 2013 Jul-Aug;27(4):283-6. doi: 10.2500/ajra.2013.27.3911. Am J Rhinol Allergy. 2013. PMID: 23883809 Review.
-
Bitter and sweet taste receptors in the respiratory epithelium in health and disease.J Mol Med (Berl). 2014 Dec;92(12):1235-44. doi: 10.1007/s00109-014-1222-6. Epub 2014 Nov 13. J Mol Med (Berl). 2014. PMID: 25391251 Free PMC article. Review.
Cited by
-
Effects of Akt Activator SC79 on Human M0 Macrophage Phagocytosis and Cytokine Production.Cells. 2024 May 24;13(11):902. doi: 10.3390/cells13110902. Cells. 2024. PMID: 38891035 Free PMC article.
-
Association between Variants of the TRPV1 Gene and Body Composition in Sub-Saharan Africans.Genes (Basel). 2024 Jun 7;15(6):752. doi: 10.3390/genes15060752. Genes (Basel). 2024. PMID: 38927688 Free PMC article.
-
The taste for health: the role of taste receptors and their ligands in the complex food/health relationship.Front Nutr. 2024 May 30;11:1396393. doi: 10.3389/fnut.2024.1396393. eCollection 2024. Front Nutr. 2024. PMID: 38873558 Free PMC article.
-
Hops bitter β-acids have antibacterial effects against sinonasal Staphylococcus aureus but also induce sinonasal cilia and mitochondrial dysfunction.Int Forum Allergy Rhinol. 2025 Mar;15(3):287-302. doi: 10.1002/alr.23487. Epub 2024 Nov 13. Int Forum Allergy Rhinol. 2025. PMID: 39533961 Free PMC article.
-
Host-microbe interactions in chronic rhinosinusitis biofilms and models for investigation.Biofilm. 2023 Sep 29;6:100160. doi: 10.1016/j.bioflm.2023.100160. eCollection 2023 Dec 15. Biofilm. 2023. PMID: 37928619 Free PMC article. Review.
References
-
- Ferencik M., Stvrtinova V. Is the immune system our sixth sense? Relation between the immune and neuroendocrine systems. Bratisl. Lek. Listy. 1997;98:187–198. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

