Inhibition of Osteoclast Differentiation and Promotion of Osteogenic Formation by Wolfiporia extensa Mycelium
- PMID: 37317624
- PMCID: PMC10580891
- DOI: 10.4014/jmb.2304.04048
Inhibition of Osteoclast Differentiation and Promotion of Osteogenic Formation by Wolfiporia extensa Mycelium
Abstract
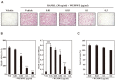
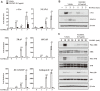
Osteoporosis, Greek for "porous bone," is a bone disease characterized by a decrease in bone strength, microarchitectural changes in the bone tissues, and an increased risk of fracture. An imbalance of bone resorption and bone formation may lead to chronic metabolic diseases such as osteoporosis. Wolfiporia extensa, known as "Bokryung" in Korea, is a fungus belonging to the family Polyporaceae and has been used as a therapeutic food against various diseases. Medicinal mushrooms, mycelium and fungi, possess approximately 130 medicinal functions, including antitumor, immunomodulating, antibacterial, hepatoprotective, and antidiabetic effects, and are therefore used to improve human health. In this study, we used osteoclast and osteoblast cell cultures treated with Wolfiporia extensa mycelium water extract (WEMWE) and investigated the effect of the fungus on bone homeostasis. Subsequently, we assessed its capacity to modulate both osteoblast and osteoclast differentiation by performing osteogenic and anti-osteoclastogenic activity assays. We observed that WEMWE increased BMP-2-stimulated osteogenesis by inducing Smad-Runx2 signal pathway axis. In addition, we found that WEMWE decreased RANKL-induced osteoclastogenesis by blocking c-Fos/NFATc1 via the inhibition of ERK and JNK phosphorylation. Our results show that WEMWE can prevent and treat bone metabolic diseases, including osteoporosis, by a biphasic activity that sustains bone homeostasis. Therefore, we suggest that WEMWE can be used as a preventive and therapeutic drug.
Keywords: Osteoporosis; Wolfiporia extensa; mycelium; osteoblasts; osteoclasts.
Conflict of interest statement
The authors have no financial conflicts of interest to declare.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

