Glucose metabolism and function of CD4+ Tregs are regulated by the TLR8/mTOR signal in an environment of SKOV3 cell growth
- PMID: 37317670
- PMCID: PMC10469653
- DOI: 10.1002/cam4.6247
Glucose metabolism and function of CD4+ Tregs are regulated by the TLR8/mTOR signal in an environment of SKOV3 cell growth
Abstract
Purpose: To investigate the role of mammalian target of rapamycin (mTOR) signal in Toll-like receptor (TLR) 8-mediated regulation of glucose metabolism and its effect on reversing immunosuppression in CD4+ regulatory T-cells (Tregs) in ovarian cancer (OC).
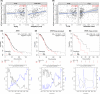
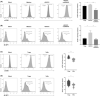
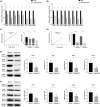
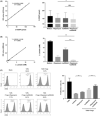
Methods: Fluorescence-activated cell sorting was used to detect the expression levels of mTOR+ and 4E-BP1+ cells in CD4+ Tregs. The prognosis and immune infiltration analysis of mTOR mRNA in OC were performed using the TIMER and Kaplan-Meier plotter database. Furthermore, real-time polymerase chain reaction (RT-PCR) and western blot (WB) were used to detect expression levels of glucose metabolism-related genes and proteins in CD4+ Tregs. Glucose uptake and glycolysis levels were detected by colorimetry, while the effects of CD4+ Tregs on the proliferation of CD4+ T-effector cells (Teffs) were evaluated by carboxyfluorescein diacetate succinimidyl ester (CFSE).
Results: mTOR expression in CD4+ Tregs was significantly higher in patients with OC compared with controls and in CD4+ Tregs than in CD4+ Teffs in OC. Additionally, the expression level of mTOR mRNA was related to prognosis and immune infiltration levels in patients with OC. Blocking the mTOR signal resulted in downregulation of glucose metabolism in CD4+ Tregs. Simultaneous inhibition of the mTOR signal while activation of the TLR8 signal had a coordinated inhibitory effect on glucose metabolism and the immunosuppressive function of CD4+ Tregs. Furthermore, the mTOR signal played an essential role in TLR8-mediated reversal of immunosuppressive function in CD4+ Tregs.
Conclusion: These findings imply that activation of the TLR8 signal inhibits glucose metabolism in CD4+ Tregs by downregulating mTOR signaling, thereby reversing the immunosuppressive function of these cells in an OC cell growth environment.
Keywords: Toll-like receptor 8; glucose metabolism; mammalian target of rapamycin; ovarian cancer; regulatory T-cells.
© 2023 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Glucose metabolism characteristics and TLR8-mediated metabolic control of CD4+ Treg cells in ovarian cancer cells microenvironment.Cell Death Dis. 2021 Jan 7;12(1):22. doi: 10.1038/s41419-020-03272-5. Cell Death Dis. 2021. PMID: 33414414 Free PMC article.
-
Interplay between mTOR and STAT5 signaling modulates the balance between regulatory and effective T cells.Immunobiology. 2015 Apr;220(4):510-7. doi: 10.1016/j.imbio.2014.10.020. Epub 2014 Oct 31. Immunobiology. 2015. PMID: 25468562
-
Ovarian Cancer Cells Promote Glycolysis Metabolism and TLR8-Mediated Metabolic Control of Human CD4+ T Cells.Front Oncol. 2020 Sep 25;10:570899. doi: 10.3389/fonc.2020.570899. eCollection 2020. Front Oncol. 2020. PMID: 33102225 Free PMC article.
-
mTOR Signaling pathway as a master regulator of memory CD8+ T-cells, Th17, and NK cells development and their functional properties.J Cell Physiol. 2019 Aug;234(8):12353-12368. doi: 10.1002/jcp.28042. Epub 2019 Feb 2. J Cell Physiol. 2019. PMID: 30710341 Review.
-
[Research progress on mTOR signaling pathway and regulatory T cell nutrition metabolic regulation mechanism].Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2024 Jan;40(1):69-73. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2024. PMID: 38246179 Review. Chinese.
Cited by
-
Senescent T Cells: The Silent Culprit in Acute Myeloid Leukemia Progression?Int J Mol Sci. 2024 Nov 22;25(23):12550. doi: 10.3390/ijms252312550. Int J Mol Sci. 2024. PMID: 39684260 Free PMC article. Review.
-
The emerging role of glycolysis and immune evasion in ovarian cancer.Cancer Cell Int. 2025 Mar 5;25(1):78. doi: 10.1186/s12935-025-03698-x. Cancer Cell Int. 2025. PMID: 40045411 Free PMC article. Review.
-
Research progress on the mechanism and markers of metabolic disorders in the occurrence and development of cognitive dysfunction after ischemic stroke.Front Endocrinol (Lausanne). 2025 Jan 22;16:1500650. doi: 10.3389/fendo.2025.1500650. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 39911922 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

