Cardiomuscular Biomarkers in the Diagnosis and Prognostication of Immune Checkpoint Inhibitor Myocarditis
- PMID: 37317858
- PMCID: PMC10527069
- DOI: 10.1161/CIRCULATIONAHA.123.062405
Cardiomuscular Biomarkers in the Diagnosis and Prognostication of Immune Checkpoint Inhibitor Myocarditis
Abstract
Background: Immune checkpoint inhibitors (ICIs) are approved for multiple cancers but can result in ICI-associated myocarditis, an infrequent but life-threatening condition. Elevations in cardiac biomarkers, specifically troponin-I (cTnI), troponin-T (cTnT), and creatine kinase (CK), are used for diagnosis. However, the association between temporal elevations of these biomarkers with disease trajectory and outcomes has not been established.
Methods: We analyzed the diagnostic accuracy and prognostic performances of cTnI, cTnT, and CK in patients with ICI myocarditis (n=60) through 1-year follow-up in 2 cardio-oncology units (APHP Sorbonne, Paris, France and Heidelberg, Germany). A total of 1751 (1 cTnT assay type), 920 (4 cTnI assay types), and 1191 CK sampling time points were available. Major adverse cardiomyotoxic events (MACE) were defined as heart failure, ventricular arrhythmia, atrioventricular or sinus block requiring pacemaker, respiratory muscle failure requiring mechanical ventilation, and sudden cardiac death. Diagnostic performance of cTnI and cTnT was also assessed in an international ICI myocarditis registry.
Results: Within 72 hours of admission, cTnT, cTnI, and CK were increased compared with upper reference limits (URLs) in 56 of 57 (98%), 37 of 42 ([88%] P=0.03 versus cTnT), and 43 of 57 ([75%] P<0.001 versus cTnT), respectively. This increased rate of positivity for cTnT (93%) versus cTnI ([64%] P<0.001) on admission was confirmed in 87 independent cases from an international registry. In the Franco-German cohort, 24 of 60 (40%) patients developed ≥1 MACE (total, 52; median time to first MACE, 5 [interquartile range, 2-16] days). The highest value of cTnT:URL within the first 72 hours of admission performed best in terms of association with MACE within 90 days (area under the curve, 0.84) than CK:URL (area under the curve, 0.70). A cTnT:URL ≥32 within 72 hours of admission was the best cut-off associated with MACE within 90 days (hazard ratio, 11.1 [95% CI, 3.2-38.0]; P<0.001), after adjustment for age and sex. cTnT was increased in all patients within 72 hours of the first MACE (23 of 23 [100%]), whereas cTnI and CK values were less than the URL in 2 of 19 (11%) and 6 of 22 (27%) of patients (P<0.001), respectively.
Conclusions: cTnT is associated with MACE and is sensitive for diagnosis and surveillance in patients with ICI myocarditis. A cTnT:URL ratio <32 within 72 hours of diagnosis is associated with a subgroup at low risk for MACE. Potential differences in diagnostic and prognostic performances between cTnT and cTnI as a function of the assays used deserve further evaluation in ICI myocarditis.
Keywords: biomarkers; drug-related side effects and adverse reactions; immune checkpoint inhibitors; myocarditis; pharmacology; troponins.
Conflict of interest statement
Figures

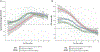


Comment in
-
Cardiac Troponins for Diagnosis and Prognostic Assessment of Immune Checkpoint Inhibitor Myocarditis and Myositis: The Emerging Importance of Peripheral Vision.Circulation. 2023 Oct 10;148(15):1135-1137. doi: 10.1161/CIRCULATIONAHA.123.065988. Epub 2023 Oct 9. Circulation. 2023. PMID: 37812655 No abstract available.
References
-
- Johnson DB, Reynolds KL, Sullivan RJ, Balko JM, Patrinely JR, Cappelli LC, Naidoo J and Moslehi JJ. Immune checkpoint inhibitor toxicities: systems-based approaches to improve patient care and research. Lancet Oncol. 2020;21:e398–e404. - PubMed
-
- Geraud A, Gougis P, Vozy A, Anquetil C, Allenbach Y, Romano E, Funck-Brentano E, Moslehi JJ, Johnson DB and Salem JE. Clinical Pharmacology and Interplay of Immune Checkpoint Agents: A Yin-Yang Balance. Annu Rev Pharmacol Toxicol. 2021;61:85–112. - PubMed
-
- Nguyen LS, Cooper LT, Kerneis M, Funck-Brentano C, Silvain J, Brechot N, Hekimian G, Ammirati E, Ben M'Barek B, Redheuil A, Gandjbakhch E, Bihan K, Lebrun-Vignes B, Ederhy S, Dolladille C, Moslehi JJ and Salem JE. Systematic analysis of drug-associated myocarditis reported in the World Health Organization pharmacovigilance database. Nat Commun. 2022;13:25. - PMC - PubMed
-
- Salem JE, Manouchehri A, Moey M, Lebrun-Vignes B, Bastarache L, Pariente A, Gobert A, Spano JP, Balko JM, Bonaca MP, Roden DM, Johnson DB and Moslehi JJ. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19:1579–1589. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

