RAB27B controls palmitoylation-dependent NRAS trafficking and signaling in myeloid leukemia
- PMID: 37317963
- PMCID: PMC10266782
- DOI: 10.1172/JCI165510
RAB27B controls palmitoylation-dependent NRAS trafficking and signaling in myeloid leukemia
Abstract
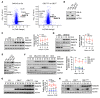
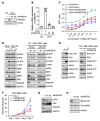
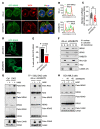
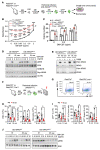
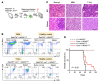
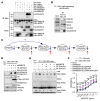
RAS mutations are among the most prevalent oncogenic drivers in cancers. RAS proteins propagate signals only when associated with cellular membranes as a consequence of lipid modifications that impact their trafficking. Here, we discovered that RAB27B, a RAB family small GTPase, controlled NRAS palmitoylation and trafficking to the plasma membrane, a localization required for activation. Our proteomic studies revealed RAB27B upregulation in CBL- or JAK2-mutated myeloid malignancies, and its expression correlated with poor prognosis in acute myeloid leukemias (AMLs). RAB27B depletion inhibited the growth of CBL-deficient or NRAS-mutant cell lines. Strikingly, Rab27b deficiency in mice abrogated mutant but not WT NRAS-mediated progenitor cell growth, ERK signaling, and NRAS palmitoylation. Further, Rab27b deficiency significantly reduced myelomonocytic leukemia development in vivo. Mechanistically, RAB27B interacted with ZDHHC9, a palmitoyl acyltransferase that modifies NRAS. By regulating palmitoylation, RAB27B controlled c-RAF/MEK/ERK signaling and affected leukemia development. Importantly, RAB27B depletion in primary human AMLs inhibited oncogenic NRAS signaling and leukemic growth. We further revealed a significant correlation between RAB27B expression and sensitivity to MEK inhibitors in AMLs. Thus, our studies presented a link between RAB proteins and fundamental aspects of RAS posttranslational modification and trafficking, highlighting future therapeutic strategies for RAS-driven cancers.
Keywords: Cancer; Cell Biology; Hematology; Signal transduction.
Figures


Comment in
-
Mechanisms for regulation of RAS palmitoylation and plasma membrane trafficking in hematopoietic malignancies.J Clin Invest. 2023 Jun 15;133(12):e171104. doi: 10.1172/JCI171104. J Clin Invest. 2023. PMID: 37317974 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

