Hand grip strength in patients with advanced cancer: A prospective study
- PMID: 37318103
- PMCID: PMC10401539
- DOI: 10.1002/jcsm.13248
Hand grip strength in patients with advanced cancer: A prospective study
Abstract
Background: Hand grip strength (HGS) is a widely used functional test for the assessment of strength and functional status in patients with cancer, in particular with cancer cachexia. The aim was to prospectively evaluate the prognostic value of HGS in patients with mostly advanced cancer with and without cachexia and to establish reference values for a European-based population.
Methods: In this prospective study, 333 patients with cancer (85% stage III/IV) and 65 healthy controls of similar age and sex were enrolled. None of the study participants had significant cardiovascular disease or active infection at baseline. Repetitive HGS assessment was performed using a hand dynamometer to measure the maximal HGS (kilograms). Presence of cancer cachexia was defined when patients had ≥5% weight loss within 6 months or when body mass index was <20.0 kg/m2 with ≥2% weight loss (Fearon's criteria). Cox proportional hazard analyses were performed to assess the relationship of maximal HGS to all-cause mortality and to determine cut-offs for HGS with the best predictive power. We also assessed associations with additional relevant clinical and functional outcome measures at baseline, including anthropometric measures, physical function (Karnofsky Performance Status and Eastern Cooperative of Oncology Group), physical activity (4-m gait speed test and 6-min walk test), patient-reported outcomes (EQ-5D-5L and Visual Analogue Scale appetite/pain) and nutrition status (Mini Nutritional Assessment).
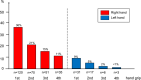
Results: The mean age was 60 ± 14 years; 163 (51%) were female, and 148 (44%) had cachexia at baseline. Patients with cancer showed 18% lower HGS than healthy controls (31.2 ± 11.9 vs. 37.9 ± 11.6 kg, P < 0.001). Patients with cancer cachexia had 16% lower HGS than those without cachexia (28.3 ± 10.1 vs. 33.6 ± 12.3 kg, P < 0.001). Patients with cancer were followed for a mean of 17 months (range 6-50), and 182 (55%) patients died during follow-up (2-year mortality rate 53%) (95% confidence interval 48-59%). Reduced maximal HGS was associated with increased mortality (per -5 kg; hazard ratio [HR] 1.19; 1.10-1.28; P < 0.0001; independently of age, sex, cancer stage, cancer entity and presence of cachexia). HGS was also a predictor of mortality in patients with cachexia (per -5 kg; HR 1.20; 1.08-1.33; P = 0.001) and without cachexia (per -5 kg; HR 1.18; 1.04-1.34; P = 0.010). The cut-off for maximal HGS with the best predictive power for poor survival was <25.1 kg for females (sensitivity 54%, specificity 63%) and <40.2 kg for males (sensitivity 69%, specificity 68%).
Conclusions: Reduced maximal HGS was associated with higher all-cause mortality, reduced overall functional status and decreased physical performance in patients with mostly advanced cancer. Similar results were found for patients with and without cancer cachexia.
Keywords: cachexia; cancer; functional assessment; hand grip strength; methodology; prognostication.
© 2023 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of Society on Sarcopenia, Cachexia and Wasting Disorders.
Conflict of interest statement
UW is supported by a Clinical Fellowship Grant from the BIH (Berlin Institute of Health) and has received speaker fees and/or contributions to congresses from Abbott, AstraZeneca, Bayer, Berlin Chemie, Bristol Myers Squibb, GE Healthcare, Pfizer, Philips and Servier, all outside the submitted work. JA reports travel support from Janssen, BMS and Alexion. UK served on advisory boards for Roche, Janssen‐Cilag, Celgene, Takeda, BMS, Gilead, Hexal, Pfizer, AstraZeneca and Pentixapharm; received clinical research support from Janssen‐Cilag, Novartis, Takeda, BMS, Roche and Pfizer; and received travel support from Roche, BMS, Gilead, Takeda, Janssen‐Cilag and Celgene. LB has received honoraria from AbbVie, Amgen, Astellas, Bristol Myers Squibb, Celgene, Daiichi Sankyo, Gilead, Hexal, Janssen, Jazz Pharmaceuticals, Menarini, Novartis, Pfizer, Roche, Sanofi and Seattle Genetics and received research support from Bayer and Jazz Pharmaceuticals. AAM reports honoraria and lecture fees from Amgen, Berlin Chemie, Daichi Sankyo, Edwards Lifesciences, Novartis and Sanofi, all not related to this work. MT reports honoraria, lecture fees and grant support from Edwards Lifesciences, AstraZeneca, Bayer, Novartis, Berlin Chemie and Daiichi Sankyo, all not related to this work. TR reports honoraria, lecture fees and grant support from Edwards Lifesciences, AstraZeneca, Bayer, Novartis, Berlin Chemie, Daiichi Sankyo, Boehringer Ingelheim, Novo Nordisk, Cardiac Dimensions and Pfizer, all not related to this work. SvH has been a paid consultant for and/or received honoraria payments from AstraZeneca, Bayer, Boehringer Ingelheim, Brahms, Chugai, Grünenthal, Helsinn, Hexal, Novartis, Pharmacosmos, Respicardia, Roche, Servier, Sorin and Vifor. He also reports research support from Amgen, AstraZeneca, Boehringer Ingelheim, Innovative Medicines Initiative (IMI) and the German Center for Cardiovascular Research (DZHK). AJSC declares having received honoraria and/or lecture fees from AstraZeneca, Boehringer Ingelheim, Menarini, Novartis, Servier, Vifor, Abbott, Actimed, Arena, Cardiac Dimensions, Corvia, CVRx, Enopace, ESN Cleer, Faraday, Impulse Dynamics, Respicardia and Viatris. SDA reports grants and personal fees from Vifor and Abbott Vascular; reports personal fees for consultancies, trial committee work and/or lectures from Actimed, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bioventrix, Brahms, Cardiac Dimensions, Cardior, Cordio, CVRx, Edwards, Farraday, Impulse Dynamics, Janssen, Novartis, Occlutech, Pfizer, Respicardia, Servier, Vectorious and V‐Wave; and declares that he is named co‐inventor of two patent applications regarding MR‐proANP (DE 102007010834 and DE 102007022367), but he does not benefit personally from the related issued patents. EJR has served as a member of the Scientific Advisory Board for Napo Pharmaceuticals, Care4ward, Actimed Therapeutics and Meter Health and has also served as a consultant for Veloxis Therapeutics, BYOMass, Takeda and Enzychem Lifesciences Pharmaceutical Company. UL reports research grant to the institution from Amgen, Bayer and Novartis and speaker or consulting honorary from AstraZeneca, Bayer, Boehringer Ingelheim, Amgen, Sanofi, Novartis and Novo Nordisk. MSA reports personal fees from Servier, outside the submitted work. All other authors declare no conflict of interest.
Figures




Similar articles
-
Associations of low hand grip strength with 1 year mortality of cancer cachexia: a multicentre observational study.J Cachexia Sarcopenia Muscle. 2021 Dec;12(6):1489-1500. doi: 10.1002/jcsm.12778. Epub 2021 Sep 20. J Cachexia Sarcopenia Muscle. 2021. PMID: 34545711 Free PMC article.
-
Hand grip strength-based cachexia index as a predictor of cancer cachexia and prognosis in patients with cancer.J Cachexia Sarcopenia Muscle. 2023 Feb;14(1):382-390. doi: 10.1002/jcsm.13139. Epub 2022 Nov 29. J Cachexia Sarcopenia Muscle. 2023. PMID: 36447437 Free PMC article.
-
Prognostic value of GLIM-defined malnutrition in combination with hand-grip strength or gait speed for the prediction of postoperative outcomes in gastric cancer patients with cachexia.BMC Cancer. 2024 Feb 23;24(1):253. doi: 10.1186/s12885-024-11880-z. BMC Cancer. 2024. PMID: 38395798 Free PMC article.
-
Physical function endpoints in cancer cachexia clinical trials: Systematic Review 1 of the cachexia endpoints series.J Cachexia Sarcopenia Muscle. 2023 Oct;14(5):1932-1948. doi: 10.1002/jcsm.13321. Epub 2023 Sep 6. J Cachexia Sarcopenia Muscle. 2023. PMID: 37671529 Free PMC article.
-
Meaningful measures in cancer cachexia: implications for practice and research.Curr Opin Support Palliat Care. 2019 Dec;13(4):323-327. doi: 10.1097/SPC.0000000000000472. Curr Opin Support Palliat Care. 2019. PMID: 31599817 Review.
Cited by
-
Exercise, cancer, and the cardiovascular system: clinical effects and mechanistic insights.Basic Res Cardiol. 2025 Feb;120(1):35-55. doi: 10.1007/s00395-024-01034-4. Epub 2024 Feb 14. Basic Res Cardiol. 2025. PMID: 38353711 Free PMC article. Review.
-
Six-Minute Walk Test Is Superior to Grip Strength as a Marker of Functional Recovery During Cancer Cachexia Rehabilitation.J Cachexia Sarcopenia Muscle. 2025 Aug;16(4):e70024. doi: 10.1002/jcsm.70024. J Cachexia Sarcopenia Muscle. 2025. PMID: 40726355 Free PMC article.
-
Prehabilitation Consultation on Self-Care and Physical Exercise in Patients Diagnosed with Abdominopelvic Cancer: Protocol of the Study.Healthcare (Basel). 2024 Jul 16;12(14):1423. doi: 10.3390/healthcare12141423. Healthcare (Basel). 2024. PMID: 39057566 Free PMC article.
-
Joint association of physical activity and the geriatric nutritional risk index with survival outcomes among cancer survivors in the United States: a population-based cohort study.Front Nutr. 2024 Dec 30;11:1483507. doi: 10.3389/fnut.2024.1483507. eCollection 2024. Front Nutr. 2024. PMID: 39807216 Free PMC article.
-
Skeletal muscle status and survival among patients with advanced biliary tract cancer.Int J Clin Oncol. 2024 Mar;29(3):297-308. doi: 10.1007/s10147-023-02466-z. Epub 2024 Feb 6. Int J Clin Oncol. 2024. PMID: 38319509 Free PMC article.
References
-
- Crawford J. What are the criteria for response to cachexia treatment? Ann Palliat Med 2019;8:43–49. - PubMed
-
- Pereira AAC, Zaia RD, Souza GHG, Luizeti BO, Andreola R, Junior AOV, et al. The correlation between hand grip strength and nutritional variables in ambulatory cancer patients. Nutr Cancer 2021;73:221–229. - PubMed
-
- Matsui R, Inaki N, Tsuji T. The impact of the preoperative hand grip strength on the long‐term outcomes after gastrectomy for advanced gastric cancer. Surg Today 2021;51:1179–1187. - PubMed
-
- Temel JS, Abernethy AP, Currow DC, Friend J, Duus EM, Yan Y, et al. Anamorelin in patients with non‐small‐cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double‐blind, phase 3 trials. Lancet Oncol 2016;17:519–531. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

