Health care registers can be instrumental for endpoint capture in clinical diabetes trials: example of microvascular complications in Swedish patients with type 2 diabetes
- PMID: 37318227
- PMCID: PMC10286550
- DOI: 10.1177/14791641231179878
Health care registers can be instrumental for endpoint capture in clinical diabetes trials: example of microvascular complications in Swedish patients with type 2 diabetes
Abstract
Aims: SMARTEST is a register-based randomized clinical trial (RRCT) that compares dapagliflozin to metformin in early-stage type 2 diabetes. The primary outcome includes progression of microvascular complications based on data from the Swedish National Diabetes Register (NDR). In this sub-study, the aim was to validate microvascular complication variables in the NDR against electronic health records (EHRs).
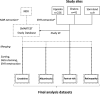
Methods: Data were extracted from EHRs of 276 SMARTEST participants with a median observation period of 3 years in the Uppsala, Örebro and Sörmland counties and compared with NDR data. Agreement was determined for all corresponding data entries as well as for progression of microvascular complications after randomization.
Results: The agreement for all corresponding data entries was 98.9% (Intraclass Correlation Coefficient 0.999) for creatinine and eGFR, 95.1% for albuminuria, 91.6% for foot-at-risk and 98.2% for retinopathy status (Kappa 0.67-0.91). The agreement for progression of microvascular complications was 98.0% for CKD stage, 98.9% for albuminuria grade, 96.3% for foot-at-risk grade and 99.6% for retinopathy grade progression (Gwet's AC1 0.96-1.00).
Conclusion: Microvascular complication variables in the NDR show good agreement with EHR data. The use of a well-established national health care registry, exemplified by the NDR, for endpoint collection in RRCTs such as SMARTEST is supported by this study.
Keywords: Type 2 diabetes; diabetes register; endpoint; microvascular complications; register-based randomized clinical trial; validation.
Conflict of interest statement
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: All authors have been involved in the conceptualization and/or conduct of the SMARTEST trial. BE and SJ are affiliated with the Swedish National Diabetes Register.
JS reports ownership in companies providing services to Itrim, Amgen, Janssen, Novo Nordisk, Eli Lilly, Boehringer Ingelheim, Bayer, Pfizer and AstraZeneca, outside the submitted work. BE reports personal fees (expert panels, lectures) from Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp and Dohme, Mundipharma, Navamedic, Novo Nordisk, RLS Global, and grants and personal fees from Sanofi, all outside the submitted work. MES has received honoraria (expert panels, lectures) from Amgen, AstraZeneca, Boehringer Ingelheim and GSK, all outside the submitted work. EG has received honoraria (advisory boards, lectures) from Bayer, Novartis, Abbvie/Allergan and Novo Nordisk, all outside the submitted work. JWE has received honoraria and research support from AstraZeneca and honoraria from Novo Nordisk, Merck Sharp and Dohme and Ilya Pharma. HN owns shares in AstraZeneca, outside the submitted work.
Figures
References
-
- Norhammar A, Bodegard J, Nystrom T, et al.Incidence, prevalence and mortality of type 2 diabetes requiring glucose-lowering treatment, and associated risks of cardiovascular complications: a nationwide study in Sweden, 2006-2013. Diabetologia 2016; 59: 1692–1701. DOI: 10.1007/s00125-016-3971-y 2016/05/18. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

