Neoadjuvant Talazoparib in Patients With Germline BRCA1/2 Mutation-Positive, Early-Stage Triple-Negative Breast Cancer: Results of a Phase II Study
- PMID: 37318349
- PMCID: PMC10546823
- DOI: 10.1093/oncolo/oyad139
Neoadjuvant Talazoparib in Patients With Germline BRCA1/2 Mutation-Positive, Early-Stage Triple-Negative Breast Cancer: Results of a Phase II Study
Abstract
Background: The undetermined efficacy of the current standard-of-care neoadjuvant treatment, anthracycline/platinum-based chemotherapy, in patients with early-stage triple-negative breast cancer (TNBC) and germline BRCA mutations emphasizes the need for biomarker-targeted treatment, such as poly(ADP-ribose) polymerase inhibitors, in this setting. This phase II, single-arm, open-label study evaluated the efficacy and safety of neoadjuvant talazoparib in patients with germline BRCA1/2-mutated early-stage TNBC.
Patients and methods: Patients with germline BRCA1/2-mutated early-stage TNBC received talazoparib 1 mg once daily for 24 weeks (0.75 mg for moderate renal impairment) followed by surgery. The primary endpoint was pathologic complete response (pCR) by independent central review (ICR). Secondary endpoints included residual cancer burden (RCB) by ICR. Safety and tolerability of talazoparib and patient-reported outcomes were assessed.
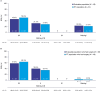
Results: Of 61 patients, 48 received ≥80% talazoparib doses, underwent surgery, and were assessed for pCR or progressed before pCR assessment and considered nonresponders. pCR rate was 45.8% (95% confidence interval [CI], 32.0%-60.6%) and 49.2% (95% CI, 36.7%-61.6%) in the evaluable and intent-to-treat (ITT) population, respectively. RCB 0/I rate was 45.8% (95% CI, 29.4%-63.2%) and 50.8% (95% CI, 35.5%-66.0%) in the evaluable and ITT population, respectively. Treatment-related adverse events (TRAE) were reported in 58 (95.1%) patients. Most common grade 3 and 4 TRAEs were anemia (39.3%) and neutropenia (9.8%). There was no clinically meaningful detriment in quality of life. No deaths occurred during the reporting period; 2 deaths due to progressive disease occurred during long-term follow-up (>400 days after first dose).
Conclusions: Neoadjuvant talazoparib monotherapy was active despite pCR rates not meeting the prespecified threshold; these rates were comparable to those observed with combination anthracycline- and taxane-based chemotherapy regimens. Talazoparib was generally well tolerated.
Clinicaltrials.gov identifier: NCT03499353.
Keywords: antineoplastic agents; neoadjuvant therapy; poly(ADP-ribose) polymerase inhibitors; triple-negative breast neoplasms.
© The Author(s) 2023. Published by Oxford University Press.
Conflict of interest statement
Jennifer K. Litton reports research support (to her institution) from AstraZeneca, EMD Serono, Genentech, GlaxoSmithKline, Medivation/Pfizer, Merck, Novartis, Pfizer, and Zenith Epigenetics; fees for participation on the speakers’ bureaus for Clinical Care Options, Med Learning Group, Medpage, Medscape, Physicians’ Education Resource, PrIME Oncology, and UpToDate; honoraria and patent/royalty payments from UpToDate; travel fees from Clinical Care Options, Med Learning Group, Medscape, and Physicians’ Education Resource; and consulting/advisory fees from AstraZeneca, Ayala Pharmaceuticals, Medivation/Pfizer, and Pfizer – all uncompensated. J. Thaddeus Beck reports research support (to his institution) from AbbVie, Amgen, Ascentage Pharma Group, AstraZeneca, Bayer, Boston Biomedical/Bristol Myers Squibb, Celgene, Daiichi Sankyo, EMD Serono, Evelo Biosciences, Genentech/Roche, GlaxoSmithKline, Hutchison, Immunomedics, Laekna Therapeutics, Lilly, Mirati Therapeutics, Nektar, Novartis, Pfizer, Polynoma, and Seattle Genetics. Jason M. Jones and Raymond Brig have nothing to disclose. Jay Andersen reports fees for participation on the speakers’ bureaus for AstraZeneca/Daiichi Sankyo, Genentech, Genomic Health, Immunomedics, Novartis, Puma Biotechnology, and Seattle Genetics; and consulting/advisory fees from AstraZeneca/Daiichi Sankyo, Athenex, Biotheranostics, Myriad, Novartis, Pfizer, Puma Biotechnology, and Seattle Genetics.
Joanne L. Blum was a physician at Texas Oncology-Baylor Charles A. Sammons Cancer Center, US Oncology Network at the time these analyses were performed and reports consulting/advisory fees and honoraria from AstraZeneca, Athenex, Inc., Biotheranostics, Immunomedics, OncLive, Pfizer, Puma Biotechnology, Research to Practice, Sanofi, Tempus, and TD2 and participation on the speakers’ bureaus for Pfizer, and Tempus. Lida A. Mina was an employee at Banner MD Anderson Cancer Center at the time these analyses were performed and has nothing to disclose. Michael Danso reports consulting/advisory fees from Immunomedics, Novartis, Pfizer, and Seattle Genetics; and honoraria from Amgen. Yuan Yuan was an employee of the City of Hope Comprehensive Cancer Center and Beckman Research Institute at the time of these analyses and reports research support from Eisai, Genentech, and Merck; consulting/advisory fees from BCI Pharma, Daiichi Sankyo, Eisai, Genentech, Gilead, Novartis, Pfizer, and Puma Biotechnology; fees for participation on the speakers’ bureaus for AstraZeneca, Daiichi Sankyo/Lilly, Eisai, Genentech, Gilead, Merck, and Pfizer; and fees for expert testimony for Novartis. Antonello Abbattista, Alexander Niyazov, Jayeta Chakrabarti, and Akos Czibere are employees of Pfizer and hold stock. Kay Noonan was an employee at Pfizer at the time of this study. William F. Symmans reports consulting/advisory fees from Almac Diagnostics and Merck; support for travel from Luminex and Merck; stock ownership in Delphi Diagnostics, Eiger BioPharmaceuticals, ISIS Pharmaceuticals, and Nuvera Biosciences; and an uncompensated relationship with Delphi Diagnostics. Melinda L. Telli reports research support (to her institution) from AbbVie, Bayer, Biothera, Calithera Biosciences, EMD Serono, Genentech, Medivation, Merck, Novartis, OncoSec, Pfizer, PharmaMar, Tesaro, and Vertex; and consulting/advisory fees from AbbVie, Aduro Biotech, AstraZeneca, Celgene, Daiichi Sankyo, G1 Therapeutics, Genentech/Roche, Guardant, Immunomedics, Lilly, Merck, Natera, Novartis, OncoSec, Pfizer, and Sanofi.
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

