Controlled Hookworm Infection for Medication-free Maintenance in Patients with Ulcerative Colitis: A Pilot, Double-blind, Randomized Control Trial
- PMID: 37318363
- PMCID: PMC11063543
- DOI: 10.1093/ibd/izad110
Controlled Hookworm Infection for Medication-free Maintenance in Patients with Ulcerative Colitis: A Pilot, Double-blind, Randomized Control Trial
Erratum in
-
Correction to: Controlled Hookworm Infection for Medication-free Maintenance in Patients with Ulcerative Colitis: A Pilot, Double-blind, Randomized Control Trial.Inflamm Bowel Dis. 2025 Oct 11:izaf235. doi: 10.1093/ibd/izaf235. Online ahead of print. Inflamm Bowel Dis. 2025. PMID: 41074405 No abstract available.
Abstract
Background: Human hookworm has been proposed as a treatment for ulcerative colitis (UC). This pilot study assessed the feasibility of a full-scale randomized control trial examining hookworm to maintain clinical remission in patients with UC.
Methods: Twenty patients with UC in disease remission (Simple Clinical Colitis Activity Index [SCCAI] ≤4 and fecal calprotectin (fCal) <100 ug/g) and only on 5-aminosalicylate received 30 hookworm larvae or placebo. Participants stopped 5-aminosalicylate after 12 weeks. Participants were monitored for up to 52 weeks and exited the study if they had a UC flare (SCCAI ≥5 and fCal ≥200 µg/g). The primary outcome was difference in rates of clinical remission at week 52. Differences were assessed for quality of life (QoL) and feasibility aspects including recruitment, safety, effectiveness of blinding, and viability of the hookworm infection.
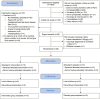
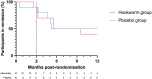
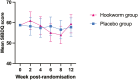
Results: At 52 weeks, 4 of 10 (40%) participants in the hookworm group and 5 of 10 (50%) participants in the placebo group had maintained clinical remission (odds ratio, 0.67; 95% CI, 0.11-3.92). Median time to flare in the hookworm group was 231 days (interquartile range [IQR], 98-365) and 259 days for placebo (IQR, 132-365). Blinding was quite successful in the placebo group (Bang's blinding index 0.22; 95% CI, -0.21 to 1) but less successful in the hookworm group (0.70; 95% CI, 0.37-1.0). Almost all participants in the hookworm group had detectable eggs in their faeces (90%; 95% CI, 0.60-0.98), and all participants in this group developed eosinophilia (peak eosinophilia 4.35 × 10^9/L; IQR, 2.80-6.68). Adverse events experienced were generally mild, and there was no significant difference in QoL.
Conclusions: A full-scale randomized control trial examining hookworm therapy as a maintenance treatment in patients with UC appears feasible.
Keywords: clinical trial; helminth; hookworm; inflammatory bowel disease; ulcerative colitis.
Plain language summary
This pilot study has shown a full-scale RCT examining hookworm therapy as maintenance therapy in patients with ulcerative colitis is feasible, safe, and will be well-tolerated.
© 2023 Crohn’s & Colitis Foundation. Published by Oxford University Press on behalf of Crohn’s & Colitis Foundation.
Conflict of interest statement
No financial disclosures or conflicts of interest.
Figures

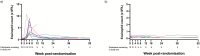


References
-
- Chang JT. Pathophysiology of inflammatory bowel diseases. Longo DL, editor. N Engl J Med. 2020 Dec 31;383(27):2652-2664. - PubMed
-
- Akdis CA. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat Rev Immunol. 2021 Nov;21(11):739-751. - PubMed
-
- Summers RW, Elliott DE, Urban JF, Thompson RA, Weinstock JV.. Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology. 2005 Apr;128(4):825-832. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

