Interactions between selectivity filter and pore helix control filter gating in the MthK channel
- PMID: 37318452
- PMCID: PMC10274084
- DOI: 10.1085/jgp.202213166
Interactions between selectivity filter and pore helix control filter gating in the MthK channel
Abstract
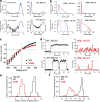
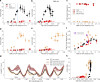
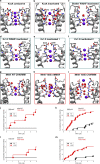
K+ channel activity can be limited by C-type inactivation, which is likely initiated in part by dissociation of K+ ions from the selectivity filter and modulated by the side chains that surround it. While crystallographic and computational studies have linked inactivation to a "collapsed" selectivity filter conformation in the KcsA channel, the structural basis for selectivity filter gating in other K+ channels is less clear. Here, we combined electrophysiological recordings with molecular dynamics simulations, to study selectivity filter gating in the model potassium channel MthK and its V55E mutant (analogous to KcsA E71) in the pore-helix. We found that MthK V55E has a lower open probability than the WT channel, due to decreased stability of the open state, as well as a lower unitary conductance. Simulations account for both of these variables on the atomistic scale, showing that ion permeation in V55E is altered by two distinct orientations of the E55 side chain. In the "vertical" orientation, in which E55 forms a hydrogen bond with D64 (as in KcsA WT channels), the filter displays reduced conductance compared to MthK WT. In contrast, in the "horizontal" orientation, K+ conductance is closer to that of MthK WT; although selectivity filter stability is lowered, resulting in more frequent inactivation. Surprisingly, inactivation in MthK WT and V55E is associated with a widening of the selectivity filter, unlike what is observed for KcsA and reminisces recent structures of inactivated channels, suggesting a conserved inactivation pathway across the potassium channel family.
© 2023 Kopec et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures






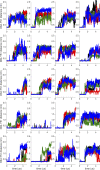
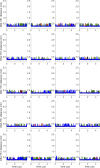





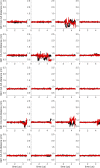
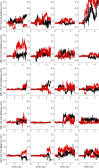
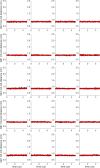
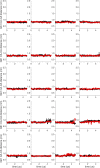
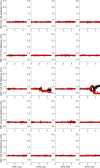



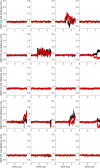



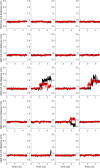
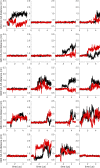








Similar articles
-
Selectivity filter ion binding affinity determines inactivation in a potassium channel.Proc Natl Acad Sci U S A. 2020 Nov 24;117(47):29968-29978. doi: 10.1073/pnas.2009624117. Epub 2020 Nov 5. Proc Natl Acad Sci U S A. 2020. PMID: 33154158 Free PMC article.
-
Initial steps of inactivation at the K+ channel selectivity filter.Proc Natl Acad Sci U S A. 2014 Apr 29;111(17):E1713-22. doi: 10.1073/pnas.1317573111. Epub 2014 Apr 14. Proc Natl Acad Sci U S A. 2014. PMID: 24733889 Free PMC article.
-
Mechanism for selectivity-inactivation coupling in KcsA potassium channels.Proc Natl Acad Sci U S A. 2011 Mar 29;108(13):5272-7. doi: 10.1073/pnas.1014186108. Epub 2011 Mar 14. Proc Natl Acad Sci U S A. 2011. PMID: 21402935 Free PMC article.
-
Diverse gating in K+ channels: differential role of the pore-helix glutamate in stabilizing the channel pore.Biochem Biophys Res Commun. 2011 Sep 16;413(1):1-4. doi: 10.1016/j.bbrc.2011.08.062. Epub 2011 Aug 22. Biochem Biophys Res Commun. 2011. PMID: 21872570 Review.
-
Small conductance calcium-activated potassium channels: from structure to function.Prog Neurobiol. 2010 Jul;91(3):242-55. doi: 10.1016/j.pneurobio.2010.03.002. Epub 2010 Mar 30. Prog Neurobiol. 2010. PMID: 20359520 Review.
Cited by
-
Functional Protein Dynamics in a Crystal.bioRxiv [Preprint]. 2024 Mar 24:2023.07.06.548023. doi: 10.1101/2023.07.06.548023. bioRxiv. 2024. Update in: Nat Commun. 2024 Apr 15;15(1):3244. doi: 10.1038/s41467-024-47473-4. PMID: 37461732 Free PMC article. Updated. Preprint.
-
Functional protein dynamics in a crystal.Nat Commun. 2024 Apr 15;15(1):3244. doi: 10.1038/s41467-024-47473-4. Nat Commun. 2024. PMID: 38622111 Free PMC article.
-
Mutation in pore-helix modulates interplay between filter gate and Ba2+ block in a Kcv channel pore.J Gen Physiol. 2024 May 6;156(5):e202313514. doi: 10.1085/jgp.202313514. Epub 2024 Apr 23. J Gen Physiol. 2024. PMID: 38652099 Free PMC article.
-
Effect of two activators on the gating of a K2P channel.Biophys J. 2024 Oct 1;123(19):3408-3420. doi: 10.1016/j.bpj.2024.08.006. Epub 2024 Aug 19. Biophys J. 2024. PMID: 39161093 Free PMC article.
-
Selectivity filter mutations shift ion permeation mechanism in potassium channels.PNAS Nexus. 2024 Jul 5;3(7):pgae272. doi: 10.1093/pnasnexus/pgae272. eCollection 2024 Jul. PNAS Nexus. 2024. PMID: 39015549 Free PMC article.
References
-
- Abraham, M.J., Murtola T., Schulz R., Páll S., Smith J.C., Hess B., and Lindahl E.. 2015. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX. 1–2:19–25. 10.1016/j.softx.2015.06.001 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

