[3 + 2] Cycloadditions of Tertiary Amine N-Oxides and Silyl Imines as an Innovative Route to 1,2-Diamines
- PMID: 37318514
- PMCID: PMC10325142
- DOI: 10.1021/acs.orglett.3c01396
[3 + 2] Cycloadditions of Tertiary Amine N-Oxides and Silyl Imines as an Innovative Route to 1,2-Diamines
Abstract
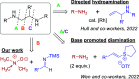
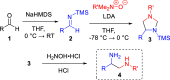
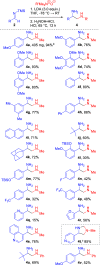
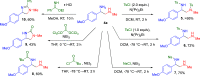
We have developed a one-pot synthetic method for producing 1,2-diamines from easily prepared and commercially available precursors through a formal umpolung process. Our method utilizes an efficient [3 + 2] cycloaddition as the key step in forming substituted 1,2-diamines in moderate to high yields. These resulting compounds can undergo subsequent transformations, demonstrating their utility as synthetic building blocks for more complex scaffolds. Finally, we propose a reasonable mechanism for this transformation using density functional theory modeling, justifying the experimental observations.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
An advance on exploring N-tert-butanesulfinyl imines in asymmetric synthesis of chiral amines.Acc Chem Res. 2008 Jul;41(7):831-40. doi: 10.1021/ar7002623. Epub 2008 Jun 6. Acc Chem Res. 2008. PMID: 18533688
-
Amine-catalyzed enantioselective 1,3-dipolar cycloadditions of aldehydes to C,N-cyclic azomethine imines.Chemistry. 2014 Apr 14;20(16):4559-62. doi: 10.1002/chem.201400333. Epub 2014 Mar 18. Chemistry. 2014. PMID: 24644273
-
Imine- and Amine-Type Macrocycles Derived from Chiral Diamines and Aromatic Dialdehydes.Molecules. 2022 Jun 25;27(13):4097. doi: 10.3390/molecules27134097. Molecules. 2022. PMID: 35807342 Free PMC article. Review.
-
Enantioselective Dynamic Exchange Reactions of Imines.J Org Chem. 2021 Sep 17;86(18):12932-12944. doi: 10.1021/acs.joc.1c01620. Epub 2021 Sep 6. J Org Chem. 2021. PMID: 34482692
-
Catalytic asymmetric synthesis of 1,2-diamines.Chem Soc Rev. 2024 Jul 29;53(15):7983-8085. doi: 10.1039/d3cs00379e. Chem Soc Rev. 2024. PMID: 38990173 Review.
Cited by
-
Diastereoselective [3 + 2] Cycloaddition between Tertiary Amine N-Oxides and Substituted Alkenes to Access 7-Azanorbornanes.Org Lett. 2024 Aug 9;26(31):6546-6550. doi: 10.1021/acs.orglett.4c02013. Epub 2024 Jul 22. Org Lett. 2024. PMID: 39038111 Free PMC article.
-
N-Oxide Insertion into LDA Dimeric Aggregates for Azomethine Ylide Formation: Explicit Solvation in Quantum Mechanical Treatment of Polarized Intermediates.J Org Chem. 2025 Mar 14;90(10):3673-3683. doi: 10.1021/acs.joc.4c03090. Epub 2025 Mar 1. J Org Chem. 2025. PMID: 40083234 Free PMC article.
-
Ring Opening of Aziridines by Pendant Sulfamates Allows for Regioselective and Stereospecific Preparation of Vicinal Diamines.J Org Chem. 2023 Nov 17;88(22):15989-16006. doi: 10.1021/acs.joc.3c01731. Epub 2023 Oct 30. J Org Chem. 2023. PMID: 37903411 Free PMC article.
References
-
- Lucet D.; Le Gall T.; Mioskowski C. The Chemistry of Vicinal Diamines. Angew. Chem., Int. Ed. 1998, 37 (19), 2580–2627. 10.1002/(SICI)1521-3773(19981016)37:19<2580::AID-ANIE2580>3.0.CO;2-L. - DOI - PubMed
- Saibabu Kotti S. R. S.; Timmons C.; Li G. Vicinal Diamino Functionalities as Privileged Structural Elements in Biologically Active Compounds and Exploitation of their Synthetic Chemistry. Chem. Biol. Drug Des. 2006, 67 (2), 101–114. 10.1111/j.1747-0285.2006.00347.x. - DOI - PubMed
- Michalson E. T.; Szmuszkovicz J.. Medicinal agents incorporating the 1,2-diamine functionality. In Progress in Drug Research; Jucker E., Ed.; Birkhäuser: Basel, Switzerland, 1989; pp 135–149,10.1007/978-3-0348-9146-2_6. - DOI - PubMed
- Weerawarna S. A.; Davis R. D.; Nelson W. L. Isothiocyanate-Substituted. kappa.-Selective Opioid Receptor Ligands Derived from N-Methyl-N-[(1S)-1-phenyl-2-(1-pyrrolidinyl) ethyl] phenylacetamide. J. Med. Chem. 1994, 37 (18), 2856–2864. 10.1021/jm00044a006. - DOI - PubMed
- Chang A.-C.; Takemori A. E.; Ojala W. H.; Gleason W. B.; Portoghese P. S. κ Opioid Receptor Selective Affinity Labels: Electrophilic Benzeneacetamides as κ-Selective Opioid Antagonists. J. Med. Chem. 1994, 37 (26), 4490–4498. 10.1021/jm00052a008. - DOI - PubMed
- Malcolmson S. J.; Li K.; Shao X. 2-Azadienes as Enamine Umpolung Synthons for the Preparation of Chiral Amines. Synlett 2019, 30 (11), 1253–1268. 10.1055/s-0037-1611770. - DOI - PMC - PubMed
- Magano J. Synthetic Approaches to the Neuraminidase Inhibitors Zanamivir (Relenza) and Oseltamivir Phosphate (Tamiflu) for the Treatment of Influenza. Chem. Rev. 2009, 109 (9), 4398–4438. 10.1021/cr800449m. - DOI - PubMed
-
- Sheshenev A. E.; Boltukhina E. V.; White A. J. P.; Hii K. K. Methylene-Bridged Bis(imidazoline)-Derived 2-Oxopyrimidinium Salts as Catalysts for Asymmetric Michael Reactions. Angew. Chem., Int. Ed. 2013, 52 (27), 6988–6991. 10.1002/anie.201300614. - DOI - PMC - PubMed
- Sutton A. E.; Richardson T. E.; Huck B. R.; Karra S. R.; Chen X.; Xiao Y.; Goutopoulos A.; Lan R.; Perrey D.; Vandeveer H. G.; Liu-Bujalski L.; Stieber F.; Hodous B. L.; Qiu H.; Jones R. C.; Heasley B.. Preparation of novel aminoazaheterocyclic carboxamides useful in the treatment of hyperproliferative diseases. WO Patent 2010093419 A1, Aug 19, 2010.
- Perha Pharmaceuticals . Synthesis of imidazolone derivatives as DYRK1, CLK1 and/or CLK4 protein kinases. EP Patent 3904354 A1, Nov 3, 2021.
- Skerlj R.; Bridger G.; Zhou Y.; Bourque E.; McEachern E.; Metz M.; Harwig C.; Li T.-S.; Yang W.; Bogucki D.; Zhu Y.; Langille J.; Veale D.; Ba T.; Bey M.; Baird I.; Kaller A.; Krumpak M.; Leitch D.; Satori M.; Vocadlo K.; Guay D.; Nan S.; Yee H.; Crawford J.; Chen G.; Wilson T.; Carpenter B.; Gauthier D.; MacFarland R.; Mosi R.; Bodart V.; Wong R.; Fricker S.; Schols D. Design of Substituted Imidazolidinylpiperidinylbenzoic Acids as Chemokine Receptor 5 Antagonists: Potent Inhibitors of R5 HIV-1 Replication. J. Med. Chem. 2013, 56 (20), 8049–8065. 10.1021/jm401101p. - DOI - PubMed
-
- Gladiali S.; Alberico E. Asymmetric transfer hydrogenation: chiral ligands and applications. Chem. Soc. Rev. 2006, 35 (3), 226–236. 10.1039/B513396C. - DOI - PubMed
- Surry D. S.; Buchwald S. L. Diamine ligands in copper-catalyzed reactions. Chem. Sci. 2010, 1 (1), 13–31. 10.1039/c0sc00107d. - DOI - PMC - PubMed
- Ikariya T.; Blacker A. J. Asymmetric Transfer Hydrogenation of Ketones with Bifunctional Transition Metal-Based Molecular Catalysts. Acc. Chem. Res. 2007, 40 (12), 1300–1308. 10.1021/ar700134q. - DOI - PubMed
- Kizirian J.-C. Chiral Tertiary Diamines in Asymmetric Synthesis. Chem. Rev. 2008, 108 (1), 140–205. 10.1021/cr040107v. - DOI - PubMed
- Chierchia M.; Law C.; Morken J. P. Nickel-Catalyzed Enantioselective Conjunctive Cross-Coupling of 9-BBN Borates. Angew. Chem., Int. Ed. 2017, 56 (39), 11870–11874. 10.1002/anie.201706719. - DOI - PMC - PubMed
-
- Yadav J. S.; Reddy B. V. S.; Rao K. V.; Raj K. S.; Prasad A. R. Indium Tribromide Catalyzed Aminolysis of Aziridines: An Efficient Synthesis of vicinal-diamines. Synthesis 2002, 2002 (8), 1061–1064. 10.1055/s-2002-31965. - DOI
- Peruncheralathan S.; Henze M.; Schneider C. Scandium Triflate Catalyzed Aminolysis of meso-Aziridines. Synlett 2007, 2007 (14), 2289–2291. 10.1055/s-2007-984920. - DOI
- Uraguchi D.; Kinoshita N.; Kizu T.; Ooi T. Synergistic Catalysis of Ionic Brønsted Acid and Photosensitizer for a Redox Neutral Asymmetric α-Coupling of N-Arylaminomethanes with Aldimines. J. Am. Chem. Soc. 2015, 137 (43), 13768–13771. 10.1021/jacs.5b09329. - DOI - PubMed
- Cardona F.; Goti A. Metal-catalysed 1,2-diamination reactions. Nat. Chem. 2009, 1 (4), 269–275. 10.1038/nchem.256. - DOI - PubMed
- Gan X.-C.; Zhang C.-Y.; Zhong F.; Tian P.; Yin L. Synthesis of chiral anti-1,2-diamine derivatives through copper(I)-catalyzed asymmetric α-addition of ketimines to aldimines. Nat. Commun. 2020, 11 (1), 4473.10.1038/s41467-020-18235-9. - DOI - PMC - PubMed
- Lee S.; Jang Y. J.; Phipps E. J. T.; Lei H.; Rovis T. Rhodium(III)-Catalyzed Three-Component 1,2-Diamination of Unactivated Terminal Alkenes. Synlett 2020, 52 (8), 1247–1252. 10.1055/s-0039-1690756. - DOI - PMC - PubMed
- Olson D. E.; Su J. Y.; Roberts D. A.; Du Bois J. Vicinal Diamination of Alkenes under Rh-Catalysis. J. Am. Chem. Soc. 2014, 136 (39), 13506–13509. 10.1021/ja506532h. - DOI - PMC - PubMed
- Darensbourg D. J.; Karroonnirun O. Ring-Opening Polymerization of Lactides Catalyzed by Natural Amino-Acid Based Zinc Catalysts. Inorg. Chem. 2010, 49 (5), 2360–2371. 10.1021/ic902271x. - DOI - PubMed
- Kondaparla S.; Soni A.; Manhas A.; Srivastava K.; Puri S. K.; Katti S. B. Synthesis and antimalarial activity of new 4-aminoquinolines active against drug resistant strains. RSC Adv. 2016, 6 (107), 105676–105689. 10.1039/C6RA14016E. - DOI - PubMed
- Nakhla J. S.; Schultz D. M.; Wolfe J. P. Palladium-catalyzed alkene carboamination reactions for the synthesis of substituted piperazines. Tetrahedron 2009, 65 (33), 6549–6570. 10.1016/j.tet.2009.04.017. - DOI - PMC - PubMed
- Shen P.-X.; Hu L.; Shao Q.; Hong K.; Yu J.-Q. Pd(II)-Catalyzed Enantioselective C(sp3)–H Arylation of Free Carboxylic Acids. J. Am. Chem. Soc. 2018, 140 (21), 6545–6549. 10.1021/jacs.8b03509. - DOI - PMC - PubMed
- Vicker N.; Burgess L.; Chuckowree I. S.; Dodd R.; Folkes A. J.; Hardick D. J.; Hancox T. C.; Miller W.; Milton J.; Sohal S.; Wang S.; Wren S. P.; Charlton P. A.; Dangerfield W.; Liddle C.; Mistry P.; Stewart A. J.; Denny W. A. Novel Angular Benzophenazines: Dual Topoisomerase I and Topoisomerase II Inhibitors as Potential Anticancer Agents. J. Med. Chem. 2002, 45 (3), 721–739. 10.1021/jm010329a. - DOI - PubMed
- Wixey J. S.; Ward B. D. Chiral calciumcatalysts for asymmetric hydroamination /cyclisation. Chem. Commun. 2011, 47 (19), 5449–5451. 10.1039/C1CC11229E. - DOI - PubMed
-
- Kumar R.; Khanna Y.; Kaushik P.; Kamal R.; Khokhar S. Recent Advancements on Metal-Free Vicinal Diamination of Alkenes: Synthetic Strategies and Mechanistic Insights. Chem. - Asian J. 2023, 18 (8), e20230001710.1002/asia.202300017. - DOI - PubMed
- Zhou P.; Shao X.; Malcolmson S. J. A Diastereodivergent and Enantioselective Approach to syn- and anti-Diamines: Development of 2-Azatrienes for Cu-Catalyzed Reductive Couplings with Imines That Furnish Allylic Amines. J. Am. Chem. Soc. 2021, 143 (34), 13999–14008. 10.1021/jacs.1c07707. - DOI - PMC - PubMed
- Tao Z.; Gilbert B. B.; Denmark S. E. Catalytic, Enantioselective syn-Diamination of Alkenes. J. Am. Chem. Soc. 2019, 141 (48), 19161–19170. 10.1021/jacs.9b11261. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

