Safety and Efficacy of Lebrikizumab in Adolescent Patients with Moderate-to-Severe Atopic Dermatitis: A 52-Week, Open-Label, Phase 3 Study
- PMID: 37318750
- PMCID: PMC10307734
- DOI: 10.1007/s13555-023-00942-y
Safety and Efficacy of Lebrikizumab in Adolescent Patients with Moderate-to-Severe Atopic Dermatitis: A 52-Week, Open-Label, Phase 3 Study
Abstract
Introduction: Atopic dermatitis (AD) is a chronic inflammatory skin disorder with limited treatment options for adolescents with moderate-to-severe disease. Lebrikizumab, a monoclonal antibody targeting interleukin (IL)-13, demonstrated clinical benefit in previous Phase 3 trials: ADvocate1 (NCT04146363), ADvocate2 (NCT04178967), and ADhere (NCT04250337). We report 52-week safety and efficacy outcomes from ADore (NCT04250350), a Phase 3, open-label study of lebrikizumab in adolescent patients with moderate-to-severe AD. The primary endpoint was to describe the proportion of patients who discontinued from study treatment because of adverse events (AEs) through the last treatment visit.
Methods: Adolescent patients (N = 206) (≥ 12 to < 18 years old, weighing ≥ 40 kg) with moderate-to-severe AD received subcutaneous lebrikizumab 500 mg loading doses at baseline and Week 2, followed by 250 mg every 2 weeks (Q2W) thereafter. Safety was monitored using reported AEs, AEs leading to treatment discontinuation, vital signs, growth assessments, and laboratory testing. Efficacy analyses included Eczema Area and Severity Index (EASI), Investigator's Global Assessment (IGA), Body Surface Area (BSA), (Children's) Dermatology Life Quality Index ((C)DLQI), and Patient-Reported Outcomes Measurement Information System (PROMIS) Anxiety, and PROMIS Depression.
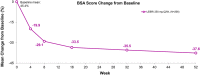
Results: 172 patients completed the treatment period. Low frequencies of SAEs (n = 5, 2.4%) and AEs leading to treatment discontinuation (n = 5, 2.4%) were reported. Overall, 134 patients (65%) reported at least one treatment-emergent AE (TEAE), most being mild or moderate in severity. In total, 62.6% achieved IGA (0,1) with ≥ 2-point improvement from baseline and 81.9% achieved EASI-75 by Week 52. The EASI mean percentage improvement from baseline to Week 52 was 86.0%. Mean BSA at baseline was 45.4%, decreasing to 8.4% by Week 52. Improvements in mean change from baseline (CFB) to Week 52 were observed in DLQI (baseline 12.3; CFB - 8.9), CDLQI (baseline 10.1; CFB - 6.5), PROMIS Anxiety (baseline 51.5; CFB - 6.3), and PROMIS Depression (baseline 49.3; CFB - 3.4) scores.
Conclusions: Lebrikizumab 250 mg Q2W had a safety profile consistent with previous trials and significantly improved AD symptoms and quality of life, with meaningful responses at Week 16 increasing by Week 52.
Trial registration: ClinicalTrials.gov identifier, NCT04250350.
Keywords: Adolescents; Efficacy; IL-13; Lebrikizumab; Moderate-to-severe atopic dermatitis; Safety.
Plain language summary
Atopic dermatitis is a chronic relapsing inflammatory skin disease that affects up to 15% of adolescents worldwide, with up to 50% suffering from moderate-to-severe disease. Signs and symptoms include dry, cracked skin; redness; itching; and painful lesions, which can negatively affect quality of life and lead to complications, including skin infections. Adolescents also report increased rates of anxiety and stress. Lebrikizumab is a novel monoclonal antibody that binds with high affinity and slow off-rate to interleukin (IL)-13, the key cytokine in atopic dermatitis, blocking the downstream effects of IL-13 with high potency. Lebrikizumab has been shown previously to improve symptoms of atopic dermatitis, including itch, skin clearance, and quality of life in ADvocate1, ADvocate2 and ADhere. The ADore study aimed to evaluate the safety and efficacy of lebrikizumab in adolescents with moderate-to-severe atopic dermatitis. Investigators recruited patients ≥ 12 to < 18 years old, weighing ≥ 40 kg, from Australia, Canada, Poland, and the US who were diagnosed with moderate-to-severe atopic dermatitis. These patients received a loading dose of 500 mg of lebrikizumab at Weeks 0 and 2, followed by 250 mg every 2 weeks for 52 weeks. The safety profile of lebrikizumab was consistent with previously published reports, with mostly mild or moderate adverse events, which did not lead to treatment discontinuation. Lebrikizumab improved skin clearance; 62.6% of patients had clear or almost clear skin by the end of the trial. Lebrikizumab also improved the patients’ quality of life. These safety and efficacy results support lebrikizumab’s role in treating adolescents with moderate-to-severe atopic dermatitis. Safety and Efficacy of Lebrikizumab in Adolescent Patients with Moderate-to-Severe Atopic Dermatitis: A 52-Week, Open-Label, Phase 3 Study (MP4 44681 KB).
© 2023. The Author(s).
Conflict of interest statement
Amy S. Paller is a consultant with honorarium from AbbVie, Acrotech, Almirall, Amgen, Amryt, Arcutis, Arena, Azitra, BioCryst, BiomX, Boeringer Ingelheim, Botanix, Bridgebio, Castle Biosciences, Catawba, Eli Lilly and Company, Exicure, Gilead, Incyte, Janssen, Kamari, LEO Pharma, Novartis, Pfizer, Pierre Fabre, RAPT, Regeneron, Sanofi/Genzyme, Seanergy, UCB, and Union. She is an investigator for AbbVie, AnaptysBio, Dermavant, Eli Lilly and Company, Incyte, Janssen, Krystal, Regeneron, and UCB. She is on the Data Safety Monitoring Board for AbbVie, Abeona, Bausch, Bristol Myers Squibb, Galderma, Inmed, and Novan. Carsten Flohr is Chief Investigator of the UK National Institute for Health Research-funded TREAT (ISRCTN15837754) and SOFTER (Clinicaltrials.gov: NCT03270566) trials as well as the UK-Irish Atopic eczema Systemic Therapy Register (A-STAR; ISRCTN11210918) and a Principal Investigator in the European Union (EU) Horizon 2020-funded BIOMAP Consortium (
Figures





References
-
- Simpson EL, Paller AS, Siegfried EC, Boguniewicz M, Sher L, Gooderham MJ, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156(1):44–56. doi: 10.1001/jamadermatol.2019.3336. - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

