Thematic Analysis of State Medicaid Buprenorphine Prior Authorization Requirements
- PMID: 37318805
- PMCID: PMC10273019
- DOI: 10.1001/jamanetworkopen.2023.18487
Thematic Analysis of State Medicaid Buprenorphine Prior Authorization Requirements
Abstract
Importance: Prior authorization (PA) requirements for buprenorphine are associated with lower provision of the medication for the treatment of opioid use disorder (OUD). While Medicare plans have eliminated PA requirements for buprenorphine, many Medicaid plans continue to require them.
Objective: To describe and classify buprenorphine coverage requirements based on thematic analysis of state Medicaid PA forms.
Design, setting, and participants: This qualitative study used a thematic analysis of 50 states' Medicaid PA forms for buprenorphine between November 2020 and March 2021. Forms were obtained from the jurisdiction's Medicaid websites and assessed for features suggesting barriers to buprenorphine access. A coding tool was developed based on a review of a sample of forms, including fields for behavioral health treatment recommendations or mandates, drug screening requirements, and dosage limitations.
Main outcomes and measures: Outcomes included PA requirements for different buprenorphine formulations. Additionally, PA forms were evaluated for various criteria such as behavioral health, drug screenings, dose-related recommendations or mandates or patient education.
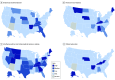
Results: Among the total of 50 US states in the analysis, most states' Medicaid plans required PA for at least 1 formulation of buprenorphine. However, the majority did not require a PA for buprenorphine-naloxone. Four key themes of coverage requirements were identified: restrictive surveillance (eg, requirements for urine drug screenings, random drug screenings, pill counts), behavioral health treatment recommendations or mandates (eg, mandatory counseling or 12-step meeting attendance), interfering with or restricting medical decision-making (eg, maximum daily dosages of 16 mg, requiring additional steps for dosages higher than 16 mg), and patient education (eg, information about adverse effects and interactions with other medications). Eleven states (22%) required urine drug screenings, 6 states (12%) required random urine drug screenings, and 4 states (8%) required pill counts. Fourteen states' forms (28%) recommended therapy, and 7 (14%) required therapy, counseling, or participation in group sessions. Eighteen states (36%) specified dosage maximums; among them, 11 (22%) required additional steps for a daily dosage higher than 16 mg.
Conclusion: In this qualitative study of state Medicaid PA requirements for buprenorphine, themes were identified that included patient surveillance with drug screenings and pill counts, behavioral health treatment recommendations or mandates, patient education, and dosing guidance. These results suggest that state Medicaid plans' buprenorphine PA requirements for OUD are in conflict with existing evidence and may negatively affect states' efforts to address the opioid overdose crisis.
Conflict of interest statement
Figures
Similar articles
-
The association of Medicare Part D prior authorization for buprenorphine-naloxone with adherence to opioid use disorder treatment guidelines in the United States.Addiction. 2022 Jan;117(1):141-150. doi: 10.1111/add.15585. Epub 2021 Jun 14. Addiction. 2022. PMID: 34033177
-
Medicaid managed care restrictions on medications for the treatment of opioid use disorder.Health Serv Res. 2025 Apr;60 Suppl 2(Suppl 2):e14394. doi: 10.1111/1475-6773.14394. Epub 2024 Oct 10. Health Serv Res. 2025. PMID: 39390740 Free PMC article.
-
Buprenorphine Use Trends Following Removal of Prior Authorization Policies for the Treatment of Opioid Use Disorder in 2 State Medicaid Programs.JAMA Health Forum. 2022 Jun 24;3(6):e221757. doi: 10.1001/jamahealthforum.2022.1757. eCollection 2022 Jun. JAMA Health Forum. 2022. PMID: 35977240 Free PMC article.
-
How do state Medicaid programs determine what substance use disorder treatment medications need prior authorization? An overview for clinicians.Addict Sci Clin Pract. 2020 Jun 29;15(1):20. doi: 10.1186/s13722-020-00194-7. Addict Sci Clin Pract. 2020. PMID: 32600402 Free PMC article. Review.
-
Toward a Typology of Office-based Buprenorphine Treatment Laws: Themes From a Review of State Laws.J Addict Med. 2022 Mar-Apr 01;16(2):192-207. doi: 10.1097/ADM.0000000000000863. J Addict Med. 2022. PMID: 34014209 Free PMC article. Review.
Cited by
-
Buprenorphine adherence among a prospective cohort of homeless-experienced adults with opioid use disorder.Drug Alcohol Depend. 2025 May 1;270:112598. doi: 10.1016/j.drugalcdep.2025.112598. Epub 2025 Feb 15. Drug Alcohol Depend. 2025. PMID: 40010041
-
Predictors of Medicaid managed care plan performance on opioid use disorder treatment quality metrics.Drug Alcohol Depend. 2025 Sep 1;274:112742. doi: 10.1016/j.drugalcdep.2025.112742. Epub 2025 Jun 2. Drug Alcohol Depend. 2025. PMID: 40482519
-
Cross-sectional examination of characteristics of higher-dose buprenorphine prescriptions during the era of illicit fentanyl.Addict Sci Clin Pract. 2025 Apr 9;20(1):33. doi: 10.1186/s13722-025-00547-0. Addict Sci Clin Pract. 2025. PMID: 40205451 Free PMC article.
-
Real-world access to buprenorphine treatment in Philadelphia: A secret shopper study.Drug Alcohol Depend. 2025 Mar 1;268:112586. doi: 10.1016/j.drugalcdep.2025.112586. Epub 2025 Jan 29. Drug Alcohol Depend. 2025. PMID: 39899919
-
Treatment for Opioid Use Disorder: Population Estimates - United States, 2022.MMWR Morb Mortal Wkly Rep. 2024 Jun 27;73(25):567-574. doi: 10.15585/mmwr.mm7325a1. MMWR Morb Mortal Wkly Rep. 2024. PMID: 38935567 Free PMC article.
References
-
- MacKinnon N, Kumar R. Prior authorization programs: a critical review of the literature. J Manag Care Pharm. 2001;7(4):297-303. doi:10.18553/jmcp.2001.7.4.297 - DOI
-
- American Medical Association . Survey: patient clinical outcomes shortchanged by prior authorization. Accessed June 15, 2022. https://www.ama-assn.org/press-center/press-releases/survey-patient-clin...
-
- American Medical Association . Issue Brief: Reports of increases in opioid-related overdose and other concerns during COVID pandemic. 2020. Accessed July 4, 2020. https://www.ama-assn.org/system/files/issue-brief-increases-in-opioid-re...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical