Renal, but not platelet or skin, extracellular vesicles decrease oxidative stress, enhance nascent peptide synthesis, and protect from ischemic renal injury
- PMID: 37318988
- PMCID: PMC10393335
- DOI: 10.1152/ajprenal.00321.2022
Renal, but not platelet or skin, extracellular vesicles decrease oxidative stress, enhance nascent peptide synthesis, and protect from ischemic renal injury
Abstract
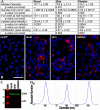
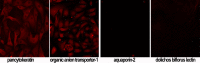
Acute kidney injury (AKI) is deadly and expensive, and specific, effective therapy remains a large unmet need. We have demonstrated the beneficial effects of transplanted adult tubular cells and extracellular vesicles (EVs; exosomes) derived from those renal cells on experimental ischemic AKI, even when administered after renal failure is established. To further examine the mechanisms of benefit with renal EVs, we tested the hypothesis that EVs from other epithelia or platelets (a rich source of EVs) might be protective, using a well-characterized ischemia-reperfusion model. When given after renal failure was present, renal EVs, but not those from skin or platelets, markedly improved renal function and histology. The differential effects allowed us to examine the mechanisms of benefit with renal EVs. We found significant decreases in oxidative stress postischemia in the renal EV-treated group with preservation of renal superoxide dismutase and catalase as well as increases in anti-inflammatory interleukin-10. In addition, we propose a novel mechanism of benefit: renal EVs enhanced nascent peptide synthesis following hypoxia in cells and in postischemic kidneys. Although EVs have been used therapeutically, these results serve as "proof of principle" to examine the mechanisms of injury and protection.NEW & NOTEWORTHY Acute kidney injury is common and deadly, yet the only approved treatment is dialysis. Thus, a better understanding of injury mechanisms and potential therapies is needed. We found that organ-specific, but not extrarenal, extracellular vesicles improved renal function and structure postischemia when given after renal failure occurred. Oxidative stress was decreased and anti-inflammatory interleukin-10 increased with renal, but not skin or platelet, exosomes. We also propose enhanced nascent peptide synthesis as a novel protective mechanism.
Keywords: acute kidney injury; exosomes; inflammation; protein biosynthesis; reactive oxygen species.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures








Similar articles
-
Comparison of cellulose, modified cellulose and synthetic membranes in the haemodialysis of patients with end-stage renal disease.Cochrane Database Syst Rev. 2001;(3):CD003234. doi: 10.1002/14651858.CD003234. Cochrane Database Syst Rev. 2001. Update in: Cochrane Database Syst Rev. 2005 Jul 20;(3):CD003234. doi: 10.1002/14651858.CD003234.pub2. PMID: 11687058 Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Pathogen-reduced platelets for the prevention of bleeding.Cochrane Database Syst Rev. 2013 Mar 28;(3):CD009072. doi: 10.1002/14651858.CD009072.pub2. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2017 Jul 30;7:CD009072. doi: 10.1002/14651858.CD009072.pub3. PMID: 23543569 Updated.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
References
-
- Susantitaphong P, Cruz DN, Cerda J, Abulfaraj M, Alqahtani F, Koulouridis I, Jaber BL; Acute Kidney Injury Advisory Group of the American Society of Nephrology. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol 8: 1482–1493, 2013. [Erratum in Clin J Am Soc Nephrol 9: 1148, 2014]. doi:10.2215/CJN.00710113. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

