Role of Strigolactones in the Host Specificity of Broomrapes and Witchweeds
- PMID: 37319019
- PMCID: PMC10504575
- DOI: 10.1093/pcp/pcad058
Role of Strigolactones in the Host Specificity of Broomrapes and Witchweeds
Abstract
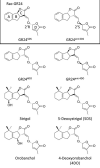
Root parasitic plants of the Orobanchaceae, broomrapes and witchweeds, pose a severe problem to agriculture in Europe, Asia and especially Africa. These parasites are totally dependent on their host for survival, and therefore, their germination is tightly regulated by host presence. Indeed, their seeds remain dormant in the soil until a host root is detected through compounds called germination stimulants. Strigolactones (SLs) are the most important class of germination stimulants. They play an important role in planta as a phytohormone and, upon exudation from the root, function in the recruitment of symbiotic arbuscular mycorrhizal fungi. Plants exude mixtures of various different SLs, possibly to evade detection by these parasites and still recruit symbionts. Vice versa, parasitic plants must only respond to the SL composition that is exuded by their host, or else risk germination in the presence of non-hosts. Therefore, parasitic plants have evolved an entire clade of SL receptors, called HTL/KAI2s, to perceive the SL cues. It has been demonstrated that these receptors each have a distinct sensitivity and specificity to the different known SLs, which possibly allows them to recognize the SL-blend characteristic of their host. In this review, we will discuss the molecular basis of SL sensitivity and specificity in these parasitic plants through HTL/KAI2s and review the evidence that these receptors contribute to host specificity of parasitic plants.
Keywords: Broomrapes; HTL/KAI2; Host specificity; Receptor-ligand specificity; Strigolactones; Witchweeds.
© The Author(s) 2023. Published by Oxford University Press on behalf of Japanese Society of Plant Physiologists.
Figures
Similar articles
-
Strigolactones as germination stimulants for root parasitic plants.Plant Cell Physiol. 2010 Jul;51(7):1095-103. doi: 10.1093/pcp/pcq055. Epub 2010 Apr 18. Plant Cell Physiol. 2010. PMID: 20403809 Free PMC article. Review.
-
A Divergent Clade KAI2 Protein in the Root Parasitic Plant Orobanche minor Is a Highly Sensitive Strigolactone Receptor and Is Involved in the Perception of Sesquiterpene Lactones.Plant Cell Physiol. 2023 Sep 15;64(9):996-1007. doi: 10.1093/pcp/pcad026. Plant Cell Physiol. 2023. PMID: 37061839 Free PMC article.
-
The mechanism of host-induced germination in root parasitic plants.Plant Physiol. 2021 Apr 23;185(4):1353-1373. doi: 10.1093/plphys/kiab043. Plant Physiol. 2021. PMID: 33793958 Free PMC article. Review.
-
Strigolactones and root infestation by plant-parasitic Striga, Orobanche and Phelipanche spp.Plant Sci. 2011 Mar;180(3):414-20. doi: 10.1016/j.plantsci.2010.11.007. Epub 2010 Nov 23. Plant Sci. 2011. PMID: 21421387 Review.
-
Highly Sensitive Strigolactone Perception by a Divergent Clade KAI2 Receptor in a Facultative Root Parasitic Plant, Phtheirospermum japonicum.Plant Cell Physiol. 2024 Dec 21;65(12):1958-1968. doi: 10.1093/pcp/pcae105. Plant Cell Physiol. 2024. PMID: 39275797
Cited by
-
Parasitic plants show striking convergence in host preference across angiosperm lineages.Ann Bot. 2025 Jul 14;135(6):1135-1146. doi: 10.1093/aob/mcae180. Ann Bot. 2025. PMID: 39447027 Free PMC article.
-
Non-specific effect of double-stranded RNAs on Egyptian broomrape (Phelipanche aegyptiaca) seed germination.Front Plant Sci. 2025 Jan 14;15:1492738. doi: 10.3389/fpls.2024.1492738. eCollection 2024. Front Plant Sci. 2025. PMID: 39877739 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

