Investigating Mycobacterium tuberculosis sufR (rv1460) in vitro and ex vivo expression and immunogenicity
- PMID: 37319185
- PMCID: PMC10270350
- DOI: 10.1371/journal.pone.0286965
Investigating Mycobacterium tuberculosis sufR (rv1460) in vitro and ex vivo expression and immunogenicity
Abstract
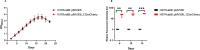
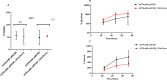
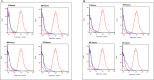
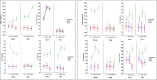
Iron is vital metal for Mycobacterium tuberculosis infection, survival, and persistence within its human host. The mobilization of sulphur (SUF) operon encodes the primary iron-sulphur (Fe-S) biogenesis system in M. tuberculosis and is induced during iron limitation and intracellular growth of M. tuberculosis, pointing to its importance during infection. To study sufR expression at single cell level during intracellular growth of M. tuberculosis a fluorescent reporter was generated by cloning a 123 bp sufR promoter region upstream of a promotorless mcherry gene in an integrating vector. Expression analysis and fluorescence measurements during in vitro culture revealed that the reporter was useful for measuring induction of the promoter but was unable to detect subsequent repression due to the stability of mCherry. During intracellular growth in THP-1 macrophages, increased fluorescence was observed in the strain harbouring the reporter relative to the control strain, however this induction was only observed in a small sub-set of the population. Since SufR levels are predicted to be elevated during infection we hypothesize that it is immunogenic and may induce an immune response in M. tuberculosis infected individuals. The immune response elicited by SufR for both whole blood assay (WBA, a short term 12-hr stimulation to characterise the production of cytokines/growth factors suggestive of an effector response) and lymphocyte proliferation assay (LPA, a longer term 7-day stimulation to see if SufR induces a memory type immune response) were low and did not show a strong immune response for the selected Luminex analytes (MCP-1, RANTES, IL-1b, IL-8, MIP-1b, IFN-g, IL-6 and MMP-9) measured in three clinical groups, namely active TB, QuantiFERON positive (QFN pos) and QFN negative (QFN neg) individuals.
Copyright: © 2023 Baatjies et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Global tuberculosis report 2019. [cited 24 Jan 2023]. Available: https://www.who.int/publications-detail-redirect/9789241565714
-
- Global tuberculosis report 2022. [cited 6 Dec 2022]. Available: https://www.who.int/publications-detail-redirect/9789240061729
-
- Grewal R, Swanepoel C, Snyders C, Isaacs S, Abayomi E. Biomarker discovery for diagnosis and treatment of tuberculosis: a role for biobanking? Journal of Biorepository Science for Applied Medicine. 2015; 47. doi: 10.2147/BSAM.S64571 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

