First virological and pathological study of Göttingen Minipigs with Dippity Pig Syndrome (DPS)
- PMID: 37319233
- PMCID: PMC10270609
- DOI: 10.1371/journal.pone.0281521
First virological and pathological study of Göttingen Minipigs with Dippity Pig Syndrome (DPS)
Abstract
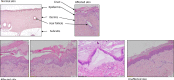
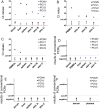
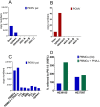
Dippity Pig Syndrome (DPS) is a well-known but rare complex of clinical signs affecting minipigs, which has not been thoroughly investigated yet. Clinically affected animals show acute appearance of red, exudating lesions across the spine. The lesions are painful, evidenced by arching of the back (dipping), and the onset of clinical signs is generally sudden. In order to understand the pathogenesis, histological and virological investigations were performed in affected and unaffected Göttingen Minipigs (GöMPs). The following DNA viruses were screened for using PCR-based methods: Porcine cytomegalovirus (PCMV), which is a porcine roseolovirus (PCMV/PRV), porcine lymphotropic herpesviruses (PLHV-1, PLHV-2, PLHV-3), porcine circoviruses (PCV1, PCV2, PCV3, PCV4), porcine parvovirus 1 (PPV1), and Torque Teno sus viruses (TTSuV1, TTSuV2). Screening was also performed for integrated porcine endogenous retroviruses (PERV-A, PERV-B, PERV-C) and recombinant PERV-A/C and their expression as well as for the RNA viruses hepatitis E virus (HEV) and SARS-CoV-2. Eight clinically affected and one unaffected GöMPs were analyzed. Additional unaffected minipigs had been analyzed in the past. The analyzed GöMPs contained PERV-A and PERV-B integrated in the genome, which are present in all pigs and PERV-C, which is present in most, but not all pigs. In one affected GöMPs recombinant PERV-A/C was detected in blood. In this animal a very high expression of PERV mRNA was observed. PCMV/PRV was found in three affected animals, PCV1 was found in three animals with DPS and in the unaffected minipig, and PCV3 was detected in two animals with DPS and in the unaffected minipig. Most importantly, in one animal only PLHV-3 was detected. It was found in the affected and unaffected skin, and in other organs. Unfortunately, PLHV-3 could not be studied in all other affected minipigs. None of the other viruses were detected and using electron microscopy, no virus particles were found in the affected skin. No porcine virus RNA with exception of PERV and astrovirus RNA were detected in the affected skin by next generation sequencing. This data identified some virus infections in GöMPs with DPS and assign a special role to PLHV-3. Since PCMV/PRV, PCV1, PCV3 and PLHV-3 were also found in unaffected animals, a multifactorial cause of DPS is suggested. However, elimination of the viruses from GöMPs may prevent DPS.
Copyright: © 2023 Jhelum et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Tynes V: Common dermatologic conditions in Vietnamese potbellied pigs—Part I: dry skin, mange, and dippity pig syndrome, Exotic Pet Pract 19961:1–2.
-
- Dijkhorst M, Stolte S, Meijer F : What is known about the dippity pig syndrome? Tijdschr Diergeneeskd 2018;143:28.
-
- McInnes EF, McKeag S: A brief review of infrequent spontaneous findings, peculiar anatomical microscopic features, and potential artifacts in Göttingen minipigs. Toxicol Pathol 2016;44:338–345. - PubMed
-
- Helke K, Nelson K, Sargeant A. Chapter 20—Animal Models in Toxicologic Research: Pig. In: Haschek WM, Rousseaux CG, Wallig MA, Bolon B (eds.) Haschek and Rousseaux’s Handbook of Toxicologic Pathology (Fourth Edition). Volume 1: Principles and Practice of Toxicologic Pathology. Academic Press, 2022, pp 751–776.
-
- Carr W, Wilbers A. Pet pig medicine 2. The sick pig. In Practice 2008;30:214–22.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

