Relapse Timing Is Associated With Distinct Evolutionary Dynamics in Diffuse Large B-Cell Lymphoma
- PMID: 37319384
- PMCID: PMC10852398
- DOI: 10.1200/JCO.23.00570
Relapse Timing Is Associated With Distinct Evolutionary Dynamics in Diffuse Large B-Cell Lymphoma
Abstract
Purpose: Diffuse large B-cell lymphoma (DLBCL) is cured in more than 60% of patients, but outcomes remain poor for patients experiencing disease progression or relapse (refractory or relapsed DLBCL [rrDLBCL]), particularly if these events occur early. Although previous studies examining cohorts of rrDLBCL have identified features that are enriched at relapse, few have directly compared serial biopsies to uncover biological and evolutionary dynamics driving rrDLBCL. Here, we sought to confirm the relationship between relapse timing and outcomes after second-line (immuno)chemotherapy and determine the evolutionary dynamics that underpin that relationship.
Patients and methods: Outcomes were examined in a population-based cohort of 221 patients with DLBCL who experienced progression/relapse after frontline treatment and were treated with second-line (immuno)chemotherapy with an intention-to-treat with autologous stem-cell transplantation (ASCT). Serial DLBCL biopsies from a partially overlapping cohort of 129 patients underwent molecular characterization, including whole-genome or whole-exome sequencing in 73 patients.
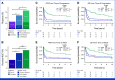
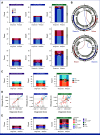
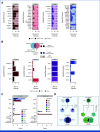
Results: Outcomes to second-line therapy and ASCT are superior for late relapse (>2 years postdiagnosis) versus primary refractory (<9 months) or early relapse (9-24 months). Diagnostic and relapse biopsies were mostly concordant for cell-of-origin classification and genetics-based subgroup. Despite this concordance, the number of mutations exclusive to each biopsy increased with time since diagnosis, and late relapses shared few mutations with their diagnostic counterpart, demonstrating a branching evolution pattern. In patients with highly divergent tumors, many of the same genes acquired new mutations independently in each tumor, suggesting that the earliest mutations in a shared precursor cell constrain tumor evolution toward the same genetics-based subgroups at both diagnosis and relapse.
Conclusion: These results suggest that late relapses commonly represent genetically distinct and chemotherapy-naïve disease and have implications for optimal patient management.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures



Update of
-
Relapse timing is associated with distinct evolutionary dynamics in DLBCL.medRxiv [Preprint]. 2023 Mar 8:2023.03.06.23286584. doi: 10.1101/2023.03.06.23286584. medRxiv. 2023. Update in: J Clin Oncol. 2023 Sep 1;41(25):4164-4177. doi: 10.1200/JCO.23.00570. PMID: 36945587 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

