Decellularized Graft for Repairing Severe Peripheral Nerve Injuries in Sheep
- PMID: 37319401
- PMCID: PMC12245264
- DOI: 10.1227/neu.0000000000002572
Decellularized Graft for Repairing Severe Peripheral Nerve Injuries in Sheep
Abstract
Background and objectives: Peripheral nerve injuries resulting in a nerve defect require surgical repair. The gold standard of autograft (AG) has several limitations, and therefore, new alternatives must be developed. The main objective of this study was to assess nerve regeneration through a long gap nerve injury (50 mm) in the peroneal nerve of sheep with a decellularized nerve allograft (DCA).
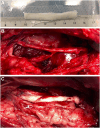
Methods: A 5-cm long nerve gap was made in the peroneal nerve of sheep and repaired using an AG or using a DCA. Functional tests were performed once a month and electrophysiology and echography evaluations at 6.5 and 9 months postsurgery. Nerve grafts were harvested at 9 months for immunohistochemical and morphological analyses.
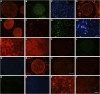
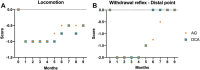
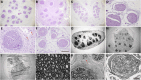
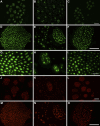
Results: The decellularization protocol completely eliminated the cells while preserving the extracellular matrix of the nerve. No significant differences were observed in functional tests of locomotion and pain response. Reinnervation of the tibialis anterior muscles occurred in all animals, with some delay in the DCA group compared with the AG group. Histology showed a preserved fascicular structure in both AG and DCA; however, the number of axons distal to the nerve graft was higher in AG than in DCA.
Conclusion: The decellularized graft assayed supported effective axonal regeneration when used to repair a 5-cm long gap in the sheep. As expected, a delay in functional recovery was observed compared with the AG because of the lack of Schwann cells.
Copyright © Congress of Neurological Surgeons 2023. All rights reserved.
Figures


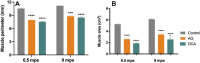
Comment in
-
Commentary: Decellularized Graft for Repairing Severe Peripheral Nerve Injuries in Sheep.Neurosurgery. 2023 Dec 1;93(6):e149-e150. doi: 10.1227/neu.0000000000002596. Epub 2023 Jul 6. Neurosurgery. 2023. PMID: 37409799 No abstract available.
Similar articles
-
Bioengineered nerve conduits and wraps for peripheral nerve repair of the upper limb.Cochrane Database Syst Rev. 2022 Dec 7;12(12):CD012574. doi: 10.1002/14651858.CD012574.pub2. Cochrane Database Syst Rev. 2022. PMID: 36477774 Free PMC article.
-
What Is the Sequence of Mechanical and Structural Failure During Stretch Injury in the Rat Median Nerve? The Neuroclasis Classification.Clin Orthop Relat Res. 2025 Jun 1;483(6):1142-1158. doi: 10.1097/CORR.0000000000003405. Epub 2025 Feb 18. Clin Orthop Relat Res. 2025. PMID: 40192591
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Biological characteristics of tissue engineered-nerve grafts enhancing peripheral nerve regeneration.Stem Cell Res Ther. 2024 Jul 18;15(1):215. doi: 10.1186/s13287-024-03827-9. Stem Cell Res Ther. 2024. PMID: 39020413 Free PMC article.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
Cited by
-
Exploring an innovative decellularization protocol for porcine nerve grafts: a translational approach to peripheral nerve repair.Front Neuroanat. 2024 Mar 19;18:1380520. doi: 10.3389/fnana.2024.1380520. eCollection 2024. Front Neuroanat. 2024. PMID: 38567289 Free PMC article.
-
Conductive and alignment-optimized porous fiber conduits with electrical stimulation for peripheral nerve regeneration.Mater Today Bio. 2024 Apr 18;26:101064. doi: 10.1016/j.mtbio.2024.101064. eCollection 2024 Jun. Mater Today Bio. 2024. PMID: 38698883 Free PMC article.
References
-
- Kasper M, Deister C, Beck F, Schmidt CE. Bench-to-bedside lessons learned: commercialization of an acellular nerve graft. Adv Healthc Mater. 2020;9(16):e2000174. - PubMed
-
- Forden J, Xu QG, Khu KJ, Midha R. A long peripheral nerve autograft model in the sheep forelimb. Neurosurgery. 2011;68(5):1354-1362. - PubMed
-
- Cai J, Peng X, Nelson KD, Eberhart R, Smith GM. Synergistic improvements in cell and axonal migration across sciatic nerve lesion gaps using bioresorbable filaments and heregulin-beta1. J Biomed Mater Res A. 2004;69(2):247-258. - PubMed
-
- Hundepool CA, Nijhuis TH, Kotsougiani D, Friedrich PF, Bishop AT, Shin AY. Optimizing decellularization techniques to create a new nerve allograft: an in vitro study using rodent nerve segments. Neurosurg Focus. 2017;42(3):e4. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

