Five-year follow-up of KEYNOTE-087: pembrolizumab monotherapy for relapsed/refractory classical Hodgkin lymphoma
- PMID: 37319435
- PMCID: PMC10624931
- DOI: 10.1182/blood.2022019386
Five-year follow-up of KEYNOTE-087: pembrolizumab monotherapy for relapsed/refractory classical Hodgkin lymphoma
Erratum in
-
Armand P, Zinzani PL, Lee HJ, et al. Five-year follow-up of KEYNOTE-087: pembrolizumab monotherapy for relapsed/refractory classical Hodgkin lymphoma. Blood. 2023;142(10):878-886.Blood. 2024 Jul 18;144(3):341. doi: 10.1182/blood.2024025229. Blood. 2024. PMID: 39023865 Free PMC article. No abstract available.
Abstract
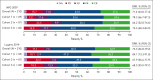
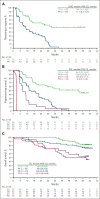
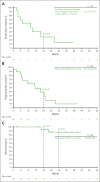
Previous analyses of the phase 2 KEYNOTE-087 (NCT02453594) trial of pembrolizumab monotherapy demonstrated effective antitumor activity with acceptable safety in patients with relapsed or refractory (R/R) classical Hodgkin lymphoma (cHL). However, long-term response durability and outcome of patients who receive a second course after treatment discontinuation after complete response (CR) remain of clinical interest. We present KEYNOTE-087 data after >5 years of median follow-up. Patients with R/R cHL and progressive disease (PD) after autologous stem cell transplantation (ASCT) and brentuximab vedotin (BV; cohort 1), salvage chemotherapy and BV without ASCT (cohort 2), or ASCT without subsequent BV (cohort 3), received pembrolizumab for ≤2 years. Patients in CR who discontinued treatment and subsequently experienced PD were eligible for second-course pembrolizumab. Primary end points were the objective response rate (ORR) using blinded central review and safety. The median follow-up was 63.7 months. ORR was 71.4% (95% confidence interval [CI], 64.8-77.4; CR, 27.6%; partial response, 43.8%). Median duration of response (DOR) was 16.6 months; median progression-free survival was 13.7 months. A quarter of responders, including half of complete responders, maintained a response for ≥4 years. Median overall survival was not achieved. Among 20 patients receiving second-course pembrolizumab, ORR for 19 evaluable patients was 73.7% (95% CI, 48.8-90.8); median DOR was 15.2 months. Any-grade treatment-related adverse events occurred in 72.9% of patients and grade 3 or 4 adverse events occurred in 12.9% of patients; no treatment-related deaths occurred. Single-agent pembrolizumab can induce durable responses, particularly in patients achieving CR. Second-course pembrolizumab frequently reinduced sustained responses after relapse from initial CR.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: P.A. reports grants or contracts to his institution from Merck, BMS, Adaptive, Genentech, IGM, and AstraZeneca within the last 24 months and Affimed, Tensha, Otsuka, and Sigma Tau; research funding from Kite within the last 24 months; consulting fees from Merck, BMS, ADC Therapeutics, GenMab, Enterome, Genentech/Roche, ATB Therapeutics, and Foresight within the last 24 months and from Pfizer, Affimed, Infinity, ADC Therapeutics, Celgene, Morphosys, Daiichi Sankyo, Miltenyi, Tessa, GenMab, C4, Enterome, Regeneron, Epizyme, and AstraZeneca; and honoraria from Merck and BMS. P.L.Z. reports honoraria from Roche, Takeda, Gilead, Sanofi, Incyte, Novartis, Merck, BMS, AstraZeneca, and Kyowa Kirin. H.J.L. reports grants or contracts from BMS, Seagen, Oncternal, Merck, and Takeda; consulting fees from Century Therapeutics; and honoraria from Aptitude Health and the Korean Society of Cardio-Oncology. N.A.J. reports consulting fees from Merck, AbbVie, BeiGene, Roche, BMS, and Kite; and honoraria from Roche, AbbVie, and BeiGene. V.R. reports advisory board roles with Gilead, Infinity, MSD, BMS, Nanostring, Incyte, Roche, and AstraZeneca; and research funding from Astex, Argen-X, and GSK. D.M. reports honoraria from MSD and BMS. T.P.V. reports grants or contracts from Merck, Takeda, Amgen, Pfizer, and Dr Reddy’s; consulting fees from Takeda, Roche, Genesis Pharma, and Novartis; honoraria from Takeda, Roche, Genesis Pharma, Merck, Novartis, Amgen, GSK, AbbVie, Integris, and AstraZeneca; and stock or stock options in Merck. A.T. reports grants or contracts from Chugai Pharmaceutical, Kyowa Kirin, Perseus Proteomics, Pfizer Japan, and Novartis Pharma K.K.; and honoraria from Chugai Pharmaceutical, Takeda Pharmaceutical, Nippon Shinyaku, Ono Pharmaceutical, and Eisai. B.v.T. reports grants or contracts from Novartis, Takeda, and Merck Sharp & Dohme; consulting fees from Allogene, BMS/Celgene, Cerus, Incyte, Miltenyi, Novartis, Pentixafarm, Roche, Amgen, Pfizer, Takeda, Merck Sharp & Dohme, and Gilead Kite; honoraria from AstraZeneca, Novartis, Roche Pharma AG, Takeda, and Merck Sharp & Dohme; and support for attending meetings and/or travel from AbbVie, AstraZeneca, Kite-Gilead, Merck Sharp & Dohme, Takeda, and Novartis. M.A.S. reports grants or contracts from BMS, Bayer, AbbVie, and AstraZeneca; and consulting fees from AstraZeneca. A.F.H. reports grants or contracts from BMS, Genentech, Merck, Seattle Genetics, AstraZeneca, Karyopharm, Kite Pharma, Gilead Sciences, AstraZeneca, and ADC Therapeutics; and consulting fees from BMS, Genentech, Merck, Seattle Genetics, AstraZeneca, Karyopharm, ADC Therapeutics, Takeda, Tubulis, Regeneron, Genmab, and Pfizer. J.L. reports employment at Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ, and is a share/stockholder or has stock options in Merck & Co Inc, Rahway, NJ. E.K. reports employment at Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc, Rahway, NJ, at the time of this analysis. S.C. reports employment at Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ, and is a share/stockholder or has stock options in Merck & Co Inc, Rahway, NJ. P.M. reports employment at Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ, and is a share/stockholder or has stock options in Merck & Co, Inc, Rahway, NJ. C.H.M. reports research funding from Merck & Co, Inc. The remaining authors declare no competing financial interests.
Figures


Comment in
-
Pembrolizumab: a key for some, but not all, HL.Blood. 2023 Sep 7;142(10):861-862. doi: 10.1182/blood.2023021050. Blood. 2023. PMID: 37676693 No abstract available.
References
-
- Hoppe RT, Advani RH, Ai WZ, et al. NCCN Guidelines® insights: Hodgkin lymphoma, version 2.2022. J Natl Compr Canc Netw. 2022;20(4):322–334. - PubMed

