Higher abatacept exposure after transplant decreases acute GVHD risk without increasing adverse events
- PMID: 37319437
- PMCID: PMC10797507
- DOI: 10.1182/blood.2023020035
Higher abatacept exposure after transplant decreases acute GVHD risk without increasing adverse events
Abstract
In the ABA2 study, the T-cell costimulation blockade agent, abatacept, was safe and effective in preventing acute graft-versus-host disease (aGVHD) after unrelated-donor hematopoietic cell transplant (HCT), leading to US Food and Drug Administration approval. Here, we performed a determination of abatacept pharmacokinetics (PK), which enabled an examination of how abatacept exposure-response relationships affected clinical outcomes. We performed a population PK analysis of IV abatacept using nonlinear mixed-effect modeling and assessed the association between abatacept exposure and key transplant outcomes. We tested the association between the trough after dose 1 (Ctrough_1) and grade (GR) 2 or 4 aGVHD (GR2-4 aGVHD) through day +100. An optimal Ctrough_1 threshold was identified via recursive partitioning and classification tree analysis. This demonstrated that abatacept PK was characterized by a 2-compartment model with first-order elimination. The ABA2 dosing regimen was based on previous work targeting a steady-state abatacept trough of 10 μg/mL. However, a higher Ctrough_1 (≥39 μg/mL, attained in ∼60% of patients on ABA2) was associated with a favorable GR2-4 aGVHD risk (hazard ratio, 0.35; 95% confidence interval, 0.19-0.65; P < .001), with a Ctrough_1 <39 μg/mL associated with GR2-4 aGVHD risk indistinguishable from placebo (P = .37). Importantly, no significant association was found between Ctrough_1 and key safety indicators, including relapse, and cytomegalovirus or Epstein-Barr virus viremia. These data demonstrate that a higher abatacept Ctrough_1 (≥39 μg/mL) was associated with a favorable GR2-4 aGVHD risk, without any observed exposure-toxicity relationships. This trial was registered at www.clinicaltrials.gov as #NCT01743131.
© 2023 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: B.R.B. receives research funding from Carisma Therapeutics; is a consultant at Magenta Therapeutics; and is a founder of Tmunity. L.S.K. is on the scientific advisory board for HiFiBio and Mammoth Biosciences; reports research funding from Magenta Therapeutics, Bluebird Bio, Bristol Myers Squibb, Novartis, Gilead, EMD-Serono, and Regeneron Pharmaceuticals; reports consulting fees from Vertex Pharmaceuticals; reports grants and personal fees from Bristol Myers Squibb during the conduct of the study; and reports grants and personal fees from Bristol Myers Squibb outside the submitted work; her financial interests with Bristol Myers Squibb are managed under an agreement with Harvard Medical School; she has a patent “Method to prevent relapse after transplant,” which is pending, and a patent “Method to prevent GVHD after transplant” with royalties paid. The remaining authors declare no competing financial interests.
Figures

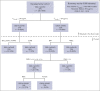
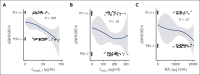
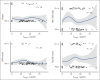
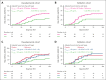
Comment in
-
GVHD: better safe than sorry.Blood. 2023 Aug 24;142(8):680-682. doi: 10.1182/blood.2023021552. Blood. 2023. PMID: 37616024 No abstract available.
References
-
- Kean LS, Burns LJ, Kou TD, et al. Improved overall survival of patients treated with abatacept in combination with a calcineurin inhibitor and methotrexate following 7/8 HLA-matched unrelated allogeneic hematopoietic stem cell transplantation: analysis of the Center for International Blood and Marrow Transplant Research Database [abstract] Blood. 2021;138(suppl 1):3912.
-
- Orencia (abatacept). Package insert. 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125118s240lbl.pdf
-
- Moreland L, Bate G, Kirkpatrick P. Abatacept. Nat Rev Drug Discov. 2006;5(3):185–186. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

