Development of active jejunal glucose absorption in broiler chickens
- PMID: 37321034
- PMCID: PMC10404788
- DOI: 10.1016/j.psj.2023.102804
Development of active jejunal glucose absorption in broiler chickens
Abstract
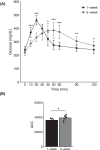
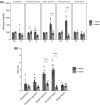
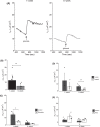
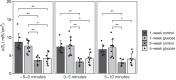
Growth in chickens, especially meat-type chickens (broilers), is extremely rapid, but studies on the regulatory mechanism of intestinal glucose absorption with growth are few, contradictory, and unclear. Here, we investigated the regulation of intestinal glucose absorption with growth in broiler chickens using oral glucose gavage, intestinal Evans blue transit, intestinal glucose absorption, scanning electron microscopy, and glucose absorption- and cell junction-related gene expression analyses. Peak blood glucose levels after oral glucose gavage occurred at 10 and 50 min in chickens at 1 wk (C1W) and 5 wk (C5W) of age, respectively. The area under the curve for glucose levels was greater for the C5W than the C1W (P = 0.035). The stain ratio in the small intestine in the C5W was lower than that in the C1W (P = 0.01), but there were no differences in the tissue regions stained with Evans blue and the migration distance of Evans blue from Meckel's diverticulum. In everted sac and Ussing chamber experiments, we observed reduced intestinal glucose uptake and electrogenic glucose absorption in the jejunum of the C5W. Phloridzin, an inhibitor of sodium/glucose cotransporter 1 (SGLT1), suppressed the glucose-induced short-circuit current in the C1W (P = 0.016) but not the C5W. Although the addition of NaCl solution stimulated the glucose-induced short-circuit current in the C1W, no differences between the treatments were observed (P = 0.056), which was also the case in the C5W. Additionally, tissue conductance was diminished in the C5W compared with that in the C1W. Moreover, in the C5W, the intestinal tract was more developed and the jejunal villi were enlarged. In conclusion, glucose absorption throughout the intestine could be greater in C5W than in C1W; however, reduced SGLT1 sensitivity, decreased ion permeability, and intestinal overdevelopment lead to decreased local glucose absorption in the jejunum with growth in broiler chickens. These data provide a detailed analysis of intestinal glucose absorption in growing broiler chickens, and can contribute to the development of novel feeds.
Keywords: age; broiler; jejunal glucose absorption; jejunal morphology; jejunal permeability.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Active transport of glucose across the jejunal epithelium decreases with age in broiler chickens.Poult Sci. 2019 Jun 1;98(6):2570-2576. doi: 10.3382/ps/pez002. Poult Sci. 2019. PMID: 30753716
-
Mechanisms underlying the inhibitory effect of the feed contaminant deoxynivalenol on glucose absorption in broiler chickens.Vet J. 2014 Oct;202(1):188-90. doi: 10.1016/j.tvjl.2014.06.012. Epub 2014 Jun 18. Vet J. 2014. PMID: 25011710
-
Effects of B-trichothecenes on luminal glucose transport across the isolated jejunal epithelium of broiler chickens.J Anim Physiol Anim Nutr (Berl). 2008 Jun;92(3):225-30. doi: 10.1111/j.1439-0396.2007.00709.x. J Anim Physiol Anim Nutr (Berl). 2008. PMID: 18477301
-
Degree of SGLT1 phosphorylation is associated with but does not determine segment-specific glucose transport features in the porcine small intestines.Physiol Rep. 2018 Jan;6(1):e13562. doi: 10.14814/phy2.13562. Physiol Rep. 2018. PMID: 29333720 Free PMC article.
-
Effects of stress simulated by dexamethasone on jejunal glucose transport in broilers.Poult Sci. 2009 Feb;88(2):330-7. doi: 10.3382/ps.2008-00257. Poult Sci. 2009. PMID: 19151348
Cited by
-
Effect of monochromatic light on self-renewal and differentiation of intestinal stem cells in broilers.Poult Sci. 2025 Jun 28;104(10):105499. doi: 10.1016/j.psj.2025.105499. Online ahead of print. Poult Sci. 2025. PMID: 40701004 Free PMC article.
-
Increasing dietary indigestible protein may exacerbate coccidiosis in broiler chickens.Anim Nutr. 2025 Jun 5;22:13-18. doi: 10.1016/j.aninu.2025.01.014. eCollection 2025 Sep. Anim Nutr. 2025. PMID: 40808933 Free PMC article.
-
Effects of Adding Bacillus coagulans BCH0 to the Diet on Growth Performance, Tissue Structures, and Gut Microbiota in Broilers.Animals (Basel). 2025 Apr 28;15(9):1243. doi: 10.3390/ani15091243. Animals (Basel). 2025. PMID: 40362058 Free PMC article.
References
-
- Amat C., Planas J.M., Moreto M. Kinetics of hexose uptake by the small and large intestine of the chicken. Am. J. Physiol. 1996;271:R1085–R1089. - PubMed
-
- Barfull A., Garriga C., Mitjans M., Planas J.M. Ontogenetic expression and regulation of Na(+)-D-glucose cotransporter in jejunum of domestic chicken. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;282:G559–G564. - PubMed
-
- Buddington R.K., Elnif J., Puchal-Gardiner A.A., Sanglid P.T. Intestinal apical amino acid absorption during development of the pig. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;280:R241–R247. - PubMed
-
- Drozdowski L., Woudstra T., Wild G., Clandindin M.T., Thomson A.B.R. The age-associated decline in the intestinal uptake of glucose is not accompanied by changes in the mRNA or protein abundance of SGLT1. Mech. Ageing Dev. 2003;124:1035–1045. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

