Early Ileocecal Resection for Crohn's Disease Is Associated With Improved Long-term Outcomes Compared With Anti-Tumor Necrosis Factor Therapy: A Population-Based Cohort Study
- PMID: 37321356
- PMCID: PMC10527197
- DOI: 10.1053/j.gastro.2023.05.051
Early Ileocecal Resection for Crohn's Disease Is Associated With Improved Long-term Outcomes Compared With Anti-Tumor Necrosis Factor Therapy: A Population-Based Cohort Study
Abstract
Background & aims: Early Crohn's disease (CD) treatment involves anti-tumor necrosis factor (TNF) agents, whereas ileocecal resection (ICR) is reserved for complicated CD or treatment failure. We compared long-term outcomes of primary ICR and anti-TNF therapy for ileocecal CD.
Methods: Using cross-linked nationwide registers, we identified all individuals diagnosed with ileal or ileocecal CD between 2003 and 2018 and treated with ICR or anti-TNF agents within 1 year of diagnosis. The primary outcome was a composite of ≥1 of the following: CD-related hospitalization, systemic corticosteroid exposure, CD-related surgery, and perianal CD. We conducted adjusted Cox's proportional hazards regression analyses and determined the cumulative risk of different treatments after primary ICR or anti-TNF therapy.
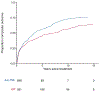
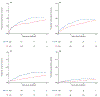
Results: Of 16,443 individuals diagnosed with CD, 1279 individuals fulfilled the inclusion criteria. Of these, 45.4% underwent ICR and 54.6% received anti-TNF. The composite outcome occurred in 273 individuals (incidence rate, 110/1000 person-years) in the ICR group and in 318 individuals (incidence rate, 202/1000 person-years) in the anti-TNF group. The risk of the composite outcome was 33% lower with ICR compared with anti-TNF (adjusted hazard ratio, 0.67; 95% confidence interval, 0.54-0.83). ICR was associated with reduced risk of systemic corticosteroid exposure and CD-related surgery, but not other secondary outcomes. The proportion of individuals on immunomodulator, anti-TNF, who underwent subsequent resection, or were on no therapy 5 years post-ICR was 46.3%, 16.8%, 1.8%, and 49.7%, respectively.
Conclusion: These data suggest that ICR may have a role as first-line therapy in CD management and challenge the current paradigm of reserving surgery for complicated CD refractory or intolerant to medications. Yet, given inherent biases associated with observational data, our findings should be interpreted and applied cautiously in clinical decision making.
Keywords: Anti-Tumor Necrosis Factor Agent; Crohn's Disease; Ileocecal Resection; Inflammatory Bowel Disease; Surgery.
Copyright © 2023 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The corresponding author confirms on behalf of all authors that there have been no involvements that might raise the question of bias in the work reported or in the conclusions, implications, or opinions stated.
MA reports no conflict of interest.
ACE reports no conflict of interest.
GP reports no conflict of interest.
RCU has served as a consultant for AbbVie, Bristol Myers Squibb, Janssen, Pfizer, and Takeda and has received research grants from AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, and Pfizer.
ASF reports no conflict of interest.
TJ reports no conflict of interest.
JFC reports receiving research grants from AbbVie, Janssen Pharmaceuticals and Takeda; receiving payment for lectures from AbbVie, Amgen, Allergan, Inc. Ferring Pharmaceuticals, Shire, and Takeda; receiving consulting fees from AbbVie, Amgen, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol Myers Squibb, Celgene Corporation, Eli Lilly, Ferring Pharmaceuticals, Galmed Research, Glaxo Smith Kline, Geneva, Iterative Scopes, Janssen Pharmaceuticals, Kaleido Biosciences, Landos, Otsuka, Pfizer, Prometheus, Sanofi, Takeda, TiGenix,; and hold stock options in Intestinal Biotech Development.
KHA reports no conflict of interest.
Figures
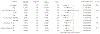

Comment in
-
First-Line Treatment Options for Ileocecal Crohn's Disease: Early Surgical Resection or Biologics?Gastroenterology. 2024 Feb;166(2):362-363. doi: 10.1053/j.gastro.2023.06.020. Epub 2023 Jul 3. Gastroenterology. 2024. PMID: 37406888 No abstract available.
-
Surgical Resection Might Be the Preferred Therapy Option in Ileocecal Crohn's Disease.Gastroenterology. 2024 Feb;166(2):361-362. doi: 10.1053/j.gastro.2023.07.020. Epub 2023 Aug 4. Gastroenterology. 2024. PMID: 37543061 No abstract available.
References
-
- Torres J, Mehandru S, Colombel JF, et al. Crohn’s disease. Lancet 2017;389:1741–1755. - PubMed
-
- Narula N, Wong ECL, Dulai PS, et al. Comparative Effectiveness of Biologies for Endoscopic Healing of the Ileum and Colon in Crohn’s Disease. Am J Gastroenterol 2022;117:1106–1117. - PubMed
-
- Ko Y, Paramsothy S, Yau Y, et al. Superior treatment persistence with ustekinumab in Crohn’s disease and vedolizumab in ulcerative colitis compared with anti-TNF biological agents: real-world registry data from the Persistence Australian National IBD Cohort (PANIC) study. Aliment Pharmacol Ther 2021;54:292–301. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

